Conductivity In Buffers . Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. The ability of any ion to. Modeling within the mean spherical approximation. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Currently, the buffer component concentration is ensured. Release of buffers throughout downstream processing include ph and conductivity. Conductivity of weak electrolytes for buffer solutions:
from chart-studio.plotly.com
Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity of weak electrolytes for buffer solutions: In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Release of buffers throughout downstream processing include ph and conductivity. Modeling within the mean spherical approximation. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. The ability of any ion to. Currently, the buffer component concentration is ensured.
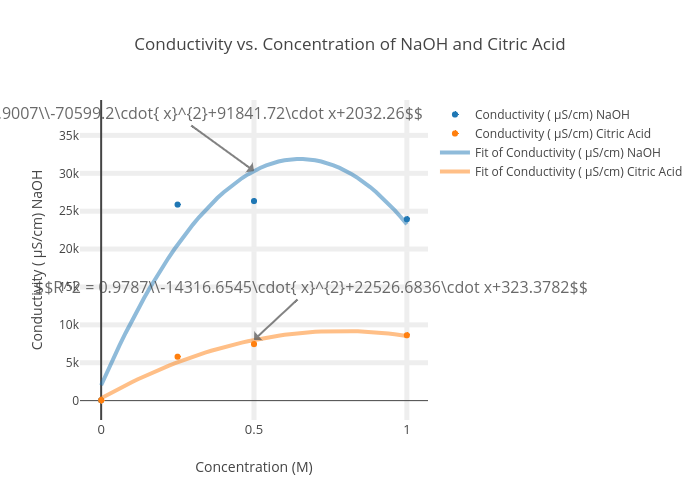
Conductivity vs. Concentration of NaOH and Citric Acid scatter chart
Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Modeling within the mean spherical approximation. The ability of any ion to. Conductivity of weak electrolytes for buffer solutions: Release of buffers throughout downstream processing include ph and conductivity. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Currently, the buffer component concentration is ensured.
From www.indomultimeter.com
Thermo Fisher Scientific Orion [011008] Conductivity Standard, 100 uS Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity of weak electrolytes for buffer solutions: In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration. Conductivity In Buffers.
From gbu-taganskij.ru
AC Conductivity Measurements Of Ultradilute Colloidal, 57 OFF Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Release of buffers throughout downstream processing include ph and conductivity. The ability of any ion to.. Conductivity In Buffers.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Modeling within the mean spherical approximation. The ability of any ion to. Conductivity of weak electrolytes. Conductivity In Buffers.
From www.youtube.com
Conductivity of Water What is Conductivity Conductivity of RO plant Conductivity In Buffers Conductivity of weak electrolytes for buffer solutions: Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. The ability of any ion to. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Currently, the buffer component concentration is ensured. Release of buffers. Conductivity In Buffers.
From www.hamiltoncompany.com
Temperature Influence of Conductivity Standard 1.3 µS/cm (Ref 238973 Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Release of buffers throughout downstream processing include ph and conductivity. The ability of any ion to.. Conductivity In Buffers.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Conductivity In Buffers Conductivity of weak electrolytes for buffer solutions: In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Release of buffers throughout downstream processing include ph and. Conductivity In Buffers.
From www.ponpe.com
Conductivity Buffer Solution ECCON1413BT 1413 µS Conductivity In Buffers Modeling within the mean spherical approximation. Currently, the buffer component concentration is ensured. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity of weak electrolytes for buffer solutions: Release of buffers throughout downstream processing include ph and conductivity. Conductivity is the measurement of the ability of a fluid. Conductivity In Buffers.
From www.fishersci.com
Conductivity Solution, OaktonBuffers and StandardsElectrochemistry Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Currently, the buffer component concentration is ensured. Modeling within the mean spherical approximation. Release of buffers. Conductivity In Buffers.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity of weak electrolytes for buffer solutions: Modeling within the mean spherical approximation. The ability of. Conductivity In Buffers.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. The ability of any ion to. Conductivity of weak electrolytes for buffer solutions: Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. In this paper, based on the influence of the number. Conductivity In Buffers.
From www.recifart.com
GHL Buffer solution conductivity 1.41ms • watertests Conductivity In Buffers Conductivity of weak electrolytes for buffer solutions: Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. The ability of any ion to. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate. Conductivity In Buffers.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Conductivity In Buffers Release of buffers throughout downstream processing include ph and conductivity. Modeling within the mean spherical approximation. The ability of any ion to. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. In this paper, based on the influence of the number of free ions and ion mobility on the. Conductivity In Buffers.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Conductivity In Buffers Modeling within the mean spherical approximation. The ability of any ion to. Conductivity of weak electrolytes for buffer solutions: Currently, the buffer component concentration is ensured. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity is the measurement of the ability of a fluid to conduct electricity via. Conductivity In Buffers.
From www.semanticscholar.org
Table 1 from Lowconductivity buffers for highsensitivity NMR Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity of weak electrolytes for buffer solutions: Release of buffers throughout downstream processing include ph and. Conductivity In Buffers.
From www.researchgate.net
Viability versus applied electrical energy. Each buffer with a final Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical. Conductivity In Buffers.
From www.researchgate.net
(PDF) Generalized Approach for Determination of Thermal Conductivity of Conductivity In Buffers Conductivity of weak electrolytes for buffer solutions: Modeling within the mean spherical approximation. Release of buffers throughout downstream processing include ph and conductivity. The ability of any ion to. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the. Conductivity In Buffers.
From www.myronl.com
Standard Solutions & Buffers Myron L® Company Conductivity In Buffers Modeling within the mean spherical approximation. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity of weak electrolytes for buffer solutions: The ability of. Conductivity In Buffers.
From chart-studio.plotly.com
Conductivity vs. Concentration of NaOH and Citric Acid scatter chart Conductivity In Buffers The ability of any ion to. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Release of buffers throughout downstream processing include ph and conductivity.. Conductivity In Buffers.
From www.slideshare.net
2012 topic 18 2 buffer solutions Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Release of buffers throughout downstream processing include ph and conductivity. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Conductivity of weak electrolytes for buffer solutions: Modeling within the mean spherical approximation.. Conductivity In Buffers.
From pdfprof.com
buffer capacity experiment procedure Conductivity In Buffers Release of buffers throughout downstream processing include ph and conductivity. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity is the measurement of the. Conductivity In Buffers.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Currently, the buffer component concentration is ensured. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Release of buffers throughout downstream processing include ph and conductivity. Modeling within the mean spherical approximation.. Conductivity In Buffers.
From www.indiamart.com
EUT18 Conductivity Buffer Solution 111.8 4 microS, Grade Standard Conductivity In Buffers In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. Conductivity of weak electrolytes for buffer solutions: Results demonstrate the impacts of factors such as ph,. Conductivity In Buffers.
From spocscientifics.com
pH & Conductivity Buffer Solutions Spoc Scientifics Conductivity In Buffers The ability of any ion to. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Modeling within the mean spherical approximation. Currently, the buffer component concentration is ensured. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical. Conductivity In Buffers.
From www.researchgate.net
Conductivity of several different buffers were measured by EUTECH Conductivity In Buffers Release of buffers throughout downstream processing include ph and conductivity. Currently, the buffer component concentration is ensured. The ability of any ion to. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of. Conductivity In Buffers.
From www.indiamart.com
LMMP30 Conductivity And pH Buffer Solution at Rs 1500.00/piece pH Conductivity In Buffers The ability of any ion to. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Modeling within the mean spherical approximation. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Release of buffers throughout downstream processing include ph and conductivity. Currently,. Conductivity In Buffers.
From www.youtube.com
Conductivity of Solutions Demonstration YouTube Conductivity In Buffers Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model. Conductivity In Buffers.
From apollo.nvu.vsc.edu
The basic concept of Conduction, heat transfer through a metal bar Conductivity In Buffers Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. The ability of any ion to. Modeling within the mean spherical approximation. Release of buffers throughout downstream processing include ph and conductivity. In this paper, based on the influence of the number of free ions and ion mobility on the. Conductivity In Buffers.
From www.acpools.com.au
Conductivity Solution (Buffer) 1413 uS/cm 250ml AC Pools Conductivity In Buffers The ability of any ion to. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Modeling within the mean spherical approximation. Currently, the buffer component concentration is ensured. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with. Conductivity In Buffers.
From webmis.highland.cc.il.us
Buffers Conductivity In Buffers Conductivity of weak electrolytes for buffer solutions: The ability of any ion to. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Currently, the buffer component concentration is ensured. Modeling within the mean spherical approximation. Release of buffers throughout downstream processing include ph and conductivity. Results demonstrate the impacts of factors such. Conductivity In Buffers.
From www.doubtnut.com
Explain the variation of molar conductivity with concentration for str Conductivity In Buffers Currently, the buffer component concentration is ensured. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. In this paper, based on the influence of the number of free ions and ion mobility. Conductivity In Buffers.
From www.researchgate.net
Effect of thermal conductivity of the buffer plate λB on heat transfer Conductivity In Buffers Modeling within the mean spherical approximation. Currently, the buffer component concentration is ensured. The ability of any ion to. Release of buffers throughout downstream processing include ph and conductivity. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the. Conductivity In Buffers.
From www.researchgate.net
Influence of buffer conductivity on binding capacity (A) Equilibrium Conductivity In Buffers Currently, the buffer component concentration is ensured. Modeling within the mean spherical approximation. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five parameters was proposed to correlate the conductivity, concentration and temperature data of electrolyte solutions at medium and high concentrations. The ability of. Conductivity In Buffers.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Conductivity In Buffers Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. Modeling within the mean spherical approximation. Release of buffers throughout downstream processing include ph and conductivity. Currently, the buffer component concentration is ensured. Conductivity of weak electrolytes for buffer solutions: The ability of any ion to. In this paper, based on the influence. Conductivity In Buffers.
From www.econogreen.com.sg
Conductivity / Buffer Solution Econo Green Conductivity In Buffers Release of buffers throughout downstream processing include ph and conductivity. Currently, the buffer component concentration is ensured. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt concentration), the level of host cell protein. The ability of any ion to. Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions.. Conductivity In Buffers.
From www.researchgate.net
30. Electrical conductivity versus concentration for the combined Conductivity In Buffers Release of buffers throughout downstream processing include ph and conductivity. Conductivity of weak electrolytes for buffer solutions: Conductivity is the measurement of the ability of a fluid to conduct electricity via its chemical ions. The ability of any ion to. Currently, the buffer component concentration is ensured. Results demonstrate the impacts of factors such as ph, conductivity (controlled by salt. Conductivity In Buffers.