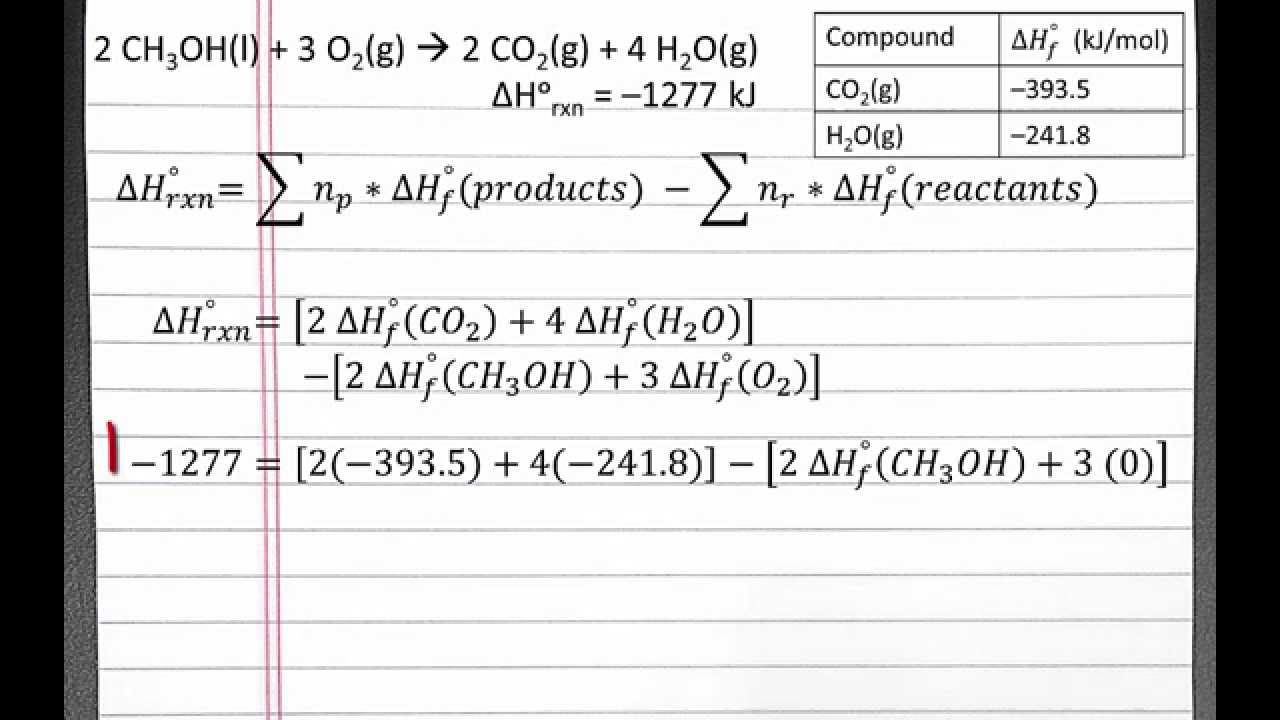
Standard Enthalpy Of Formation Is Zero For All The Following Except . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Why is that a convention? The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The symbol for the standard enthalpy of formation is: Oxygen (the element) at standard state is o 2. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is zero for elements in their standard states. The standard enthalpy of formation of any element in its standard state is zero by definition. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at.
from classhirsch.z21.web.core.windows.net
All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3. Oxygen (the element) at standard state is o 2. The symbol for the standard enthalpy of formation is: Why is that a convention?
Standard Enthalpy Of Formation Chart
Standard Enthalpy Of Formation Is Zero For All The Following Except The symbol for the standard enthalpy of formation is: The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is zero for elements in their standard states. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. Why is that a convention? The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Oxygen (the element) at standard state is o 2.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation is zero for elements in their standard states. The symbol for the standard enthalpy of formation is: For example, although oxygen can exist as ozone (o 3. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. All chemical reactions involve a. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From askfilo.com
The enthalpy of formation of all elements in their standard state is.. Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Is Zero For All The Following Except Why is that a convention? The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Oxygen (the element) at standard state is o 2. The symbol for the standard enthalpy of formation is: For example, although oxygen can exist as ozone (o 3. All chemical reactions involve. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Is Zero For All The Following Except For example, although oxygen can exist as ozone (o 3. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Why is that a. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From classhirsch.z21.web.core.windows.net
Standard Enthalpy Of Formation Chart Standard Enthalpy Of Formation Is Zero For All The Following Except The symbol for the standard enthalpy of formation is: For example, although oxygen can exist as ozone (o 3. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. Why is that a convention? The standard enthalpy of formation is zero for elements in their standard states.. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Is Zero For All The Following Except 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form.. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Is Zero For All The Following Except Why is that a convention? Oxygen (the element) at standard state is o 2. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Is Zero For All The Following Except For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard enthalpy of formation is: The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From askfilo.com
Consider the following. (a) Standard enthalpy of formation of Br2 (I) is Standard Enthalpy Of Formation Is Zero For All The Following Except Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. Oxygen (the element) at standard state is o 2. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation is zero for elements in their standard states. All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation is zero for elements in their standard states. Why is that a convention? The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Study with quizlet and memorize flashcards. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation of any element in its standard state is zero by definition. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation is zero for elements in their standard states. Why is that a convention? Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Is Zero For All The Following Except The symbol for the standard enthalpy of formation is: Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is zero for elements in their standard states. All chemical reactions involve a change in enthalpy (defined as the heat produced. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.chegg.com
Solved Select which of the following standard enthalpy of Standard Enthalpy Of Formation Is Zero For All The Following Except Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The symbol for the standard enthalpy of formation is: For example, although oxygen can exist as ozone (o 3. 193 rows in chemistry. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol for the standard enthalpy of formation is: The standard enthalpy of. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From kunduz.com
[ANSWERED] 61 180 Standard molar enthalpy of formation at 298 K is zero Standard Enthalpy Of Formation Is Zero For All The Following Except The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. Why is that a convention? The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The same is true other other gaseous elements, such. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Oxygen (the element) at standard state is o 2. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is zero for elements in their standard states. Why. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is Zero For All The Following Except The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. The standard enthalpy of formation is zero for elements in their standard states. The. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Is Zero For All The Following Except Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation is zero for elements in their standard states. The. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. The standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and Standard Enthalpy Of Formation Is Zero For All The Following Except Why is that a convention? The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is zero for elements in their standard states. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation of any element in its standard state is zero by definition. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.chegg.com
Solved All of the following have a standard enthalpy of Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The symbol for the standard enthalpy of formation is: Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. The same is true other. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Study with quizlet and memorize flashcards containing terms like. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Is Zero For All The Following Except The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a.. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From slideplayer.com
Enthalpy of formation ΔHºf ppt download Standard Enthalpy Of Formation Is Zero For All The Following Except 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Oxygen (the element) at standard state is o 2. For example, although oxygen can. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Is Zero For All The Following Except The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Is Zero For All The Following Except Oxygen (the element) at standard state is o 2. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.numerade.com
SOLVEDWhich of the following standard enthalpy of formation values is Standard Enthalpy Of Formation Is Zero For All The Following Except Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation value of zero at. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Is Zero For All The Following Except 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Oxygen (the element) at standard state is o 2. The symbol for the standard enthalpy of formation is: Study with quizlet and memorize flashcards containing terms like which of the following has a standard enthalpy of formation. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Enthalpy Of Formation Is Zero For All The Following Except The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is zero for elements in their standard states. For. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Is Zero For All The Following Except 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Oxygen (the element) at. Standard Enthalpy Of Formation Is Zero For All The Following Except.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Is Zero For All The Following Except All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is zero for elements in their standard states. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the. Standard Enthalpy Of Formation Is Zero For All The Following Except.