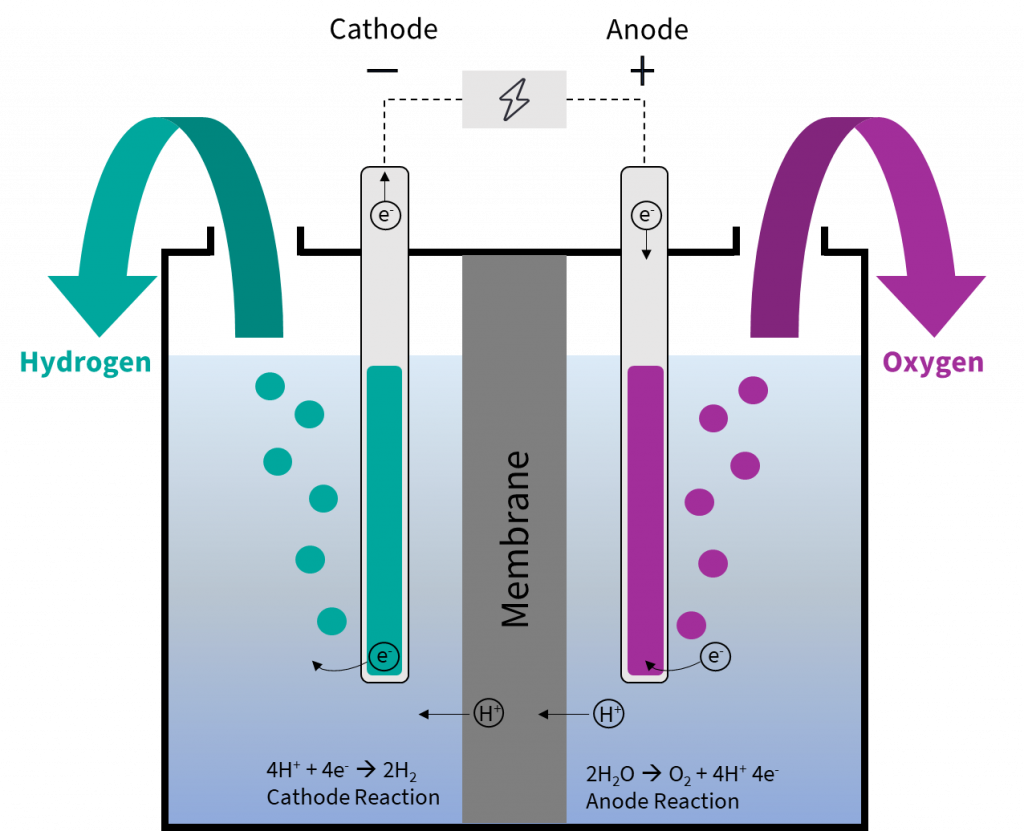
Why Is Platinum Used In Electrolysis . as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. Because of its stability, like au, it is often used to make jewelry. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. pt is a noble metal that is very stable and resistant to corrosion. You can order pt as a wire of various diameters or foil with different thicknesses. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed.
from ptx-hub.org
as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. You can order pt as a wire of various diameters or foil with different thicknesses. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. Because of its stability, like au, it is often used to make jewelry. pt is a noble metal that is very stable and resistant to corrosion. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite.
Water electrolysis explained the basis for most PowertoX processes
Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. You can order pt as a wire of various diameters or foil with different thicknesses. Because of its stability, like au, it is often used to make jewelry. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. pt is a noble metal that is very stable and resistant to corrosion. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite.
From www.youtube.com
Electrochemistry Electrolysis, Electrolytic Cell & Electroplating Why Is Platinum Used In Electrolysis these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. as pointed out. Why Is Platinum Used In Electrolysis.
From www.american-scientific.com
Platinum Electrode, Pair (for the Hoffman Electrolysis) American Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. Because of its stability, like au, it is often used to make jewelry. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. Electrolysis is the process of passing the electric current in. Why Is Platinum Used In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Why Is Platinum Used In Electrolysis Electrolysis is the process of passing the electric current in a solution that will chemically affect the. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. pt is a. Why Is Platinum Used In Electrolysis.
From www.knowledgeboat.com
Chapter 6 Electrolysis Selina Solutions Concise Chemistry Class 10 Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. You can order pt as a wire of various diameters or foil with different thicknesses. these electrodes are often made of. Why Is Platinum Used In Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Why Is Platinum Used In Electrolysis As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert material such as stainless steel,. Why Is Platinum Used In Electrolysis.
From askfilo.com
Electolysis of CuSO4 solution using platinum electrodes Electrolysis of.. Why Is Platinum Used In Electrolysis Because of its stability, like au, it is often used to make jewelry. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. these electrodes are often made of an. Why Is Platinum Used In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. You can order pt as a wire of various diameters or foil with different thicknesses. pt is a noble metal that is very stable and resistant to. Why Is Platinum Used In Electrolysis.
From h2life.org
Electrolysis of water with platinum H2Life Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert material such as stainless. Why Is Platinum Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any. Why Is Platinum Used In Electrolysis.
From circuitcoffeegirl89t1.z14.web.core.windows.net
Cathode Charge In Electrolysis Why Is Platinum Used In Electrolysis as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. pt is a noble metal that is very stable and resistant to corrosion. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. You can order pt as a wire of. Why Is Platinum Used In Electrolysis.
From www.ravindraheraeus.com
Platinum Electrodes Ravindra Heraeus Why Is Platinum Used In Electrolysis According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. You can order pt as a wire of various diameters or foil with different thicknesses. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. As an electrode, it is used to. Why Is Platinum Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Why Is Platinum Used In Electrolysis as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. Electrolysis is the process of passing. Why Is Platinum Used In Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. the most important difference is found. Why Is Platinum Used In Electrolysis.
From www.researchgate.net
(PDF) Platinum Group Metalfree Catalysts for Hydrogen Evolution Why Is Platinum Used In Electrolysis As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. You can order pt as a wire of various diameters or foil with different thicknesses. the most important difference is found in the open circuit potential,. Why Is Platinum Used In Electrolysis.
From fphoto.photoshelter.com
electrolysis potassium iodide science Fundamental Photographs The Why Is Platinum Used In Electrolysis Because of its stability, like au, it is often used to make jewelry. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. as pointed out in the other answer, platinum makes the oxidation of. Why Is Platinum Used In Electrolysis.
From tanaka-preciousmetals.com
Principles of ProtonExchange Membrane Fuel Cells and Role of Platinum Why Is Platinum Used In Electrolysis as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. pt is a noble metal that is very stable and resistant to corrosion. Because. Why Is Platinum Used In Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. the most important difference is found in the open circuit potential, that. Why Is Platinum Used In Electrolysis.
From dxomkdlos.blob.core.windows.net
Why Do Catalytic Converters Have Platinum at Monica Meador blog Why Is Platinum Used In Electrolysis these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. Because of its stability, like au, it is often used to. Why Is Platinum Used In Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Why Is Platinum Used In Electrolysis the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. pt is a noble metal that is very stable and resistant to corrosion. . Why Is Platinum Used In Electrolysis.
From brainly.in
electrolysis of acidified water using platinum electrodes gives Why Is Platinum Used In Electrolysis Electrolysis is the process of passing the electric current in a solution that will chemically affect the. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. pt is a noble metal that is very stable and resistant to corrosion. the most important difference is found in the open circuit potential,. Why Is Platinum Used In Electrolysis.
From www.youtube.com
DG Pathshala Electrolysis of CuSO4 Using Platinum ElectrodesClass Why Is Platinum Used In Electrolysis You can order pt as a wire of various diameters or foil with different thicknesses. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. pt is a noble metal that is very stable and resistant to corrosion. as pointed out in the other answer, platinum makes the oxidation of hydrogen. Why Is Platinum Used In Electrolysis.
From keystagewiki.com
Extracting Metals by Electrolysis Key Stage Wiki Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. You can order pt as a wire of various diameters or foil with different thicknesses. Because of its stability, like au, it is often used to make jewelry. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. these. Why Is Platinum Used In Electrolysis.
From mungfali.com
Electrolysis Process Diagram Why Is Platinum Used In Electrolysis the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. Because of its stability, like au, it is often used to make jewelry. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. Electrolysis is. Why Is Platinum Used In Electrolysis.
From www.youtube.com
Electrolysis of Aqueous NaCl with platinum electrodes Electrolysis Why Is Platinum Used In Electrolysis as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. You can order pt as a wire of various diameters or foil with different thicknesses. pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert. Why Is Platinum Used In Electrolysis.
From www.youtube.com
Electrolysis The Basics GCSE Science Chemistry Get To Know Why Is Platinum Used In Electrolysis Because of its stability, like au, it is often used to make jewelry. You can order pt as a wire of various diameters or foil with different thicknesses. As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. the most important difference is found in the open circuit potential, that it is almost. Why Is Platinum Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Why Is Platinum Used In Electrolysis as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. pt is a noble metal that is very stable and resistant to corrosion. Because of its stability, like au, it is often used to make jewelry. According to the characteristics of electrolyzed oxidizing water (eow), it can. Why Is Platinum Used In Electrolysis.
From www.nagwa.com
Question Video Identifying a Major Disadvantage of Using Platinum Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. You can order pt as a wire of various diameters or foil with different thicknesses. Electrolysis is the process of passing the electric current in a solution that will chemically. Why Is Platinum Used In Electrolysis.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Why Is Platinum Used In Electrolysis Because of its stability, like au, it is often used to make jewelry. You can order pt as a wire of various diameters or foil with different thicknesses. pt is a noble metal that is very stable and resistant to corrosion. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. As an. Why Is Platinum Used In Electrolysis.
From www.toppr.com
On electrolysis using platinum electrodes dilute nitric acid yields Why Is Platinum Used In Electrolysis As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. as pointed out in the other answer, platinum makes the. Why Is Platinum Used In Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Why Is Platinum Used In Electrolysis these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. pt is a noble metal that is very stable and resistant to corrosion. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. as pointed out in the. Why Is Platinum Used In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Why Is Platinum Used In Electrolysis As an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. You can order pt as a wire of various diameters or foil with different thicknesses. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. pt is a noble metal that is very stable and resistant. Why Is Platinum Used In Electrolysis.
From askfilo.com
III. Electrolysis of copper sulphate solution using platinum anode and co.. Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. these electrodes are often made of an inert material such as stainless steel, platinum, or graphite. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. As an electrode, it is used. Why Is Platinum Used In Electrolysis.
From www.vrogue.co
Introduction To Electrolysis Redox Reactions And Elec vrogue.co Why Is Platinum Used In Electrolysis Because of its stability, like au, it is often used to make jewelry. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. As an electrode, it is used to oxidize. Why Is Platinum Used In Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of acidified water with Platinum electrodes Why Is Platinum Used In Electrolysis You can order pt as a wire of various diameters or foil with different thicknesses. as pointed out in the other answer, platinum makes the oxidation of hydrogen gas to protons occur faster at any given. the most important difference is found in the open circuit potential, that it is almost doubled in the case of the ti/ru.. Why Is Platinum Used In Electrolysis.
From 2012books.lardbucket.org
Electrolysis Why Is Platinum Used In Electrolysis pt is a noble metal that is very stable and resistant to corrosion. According to the characteristics of electrolyzed oxidizing water (eow), it can be divided into acidic electrolyzed. Electrolysis is the process of passing the electric current in a solution that will chemically affect the. You can order pt as a wire of various diameters or foil with. Why Is Platinum Used In Electrolysis.