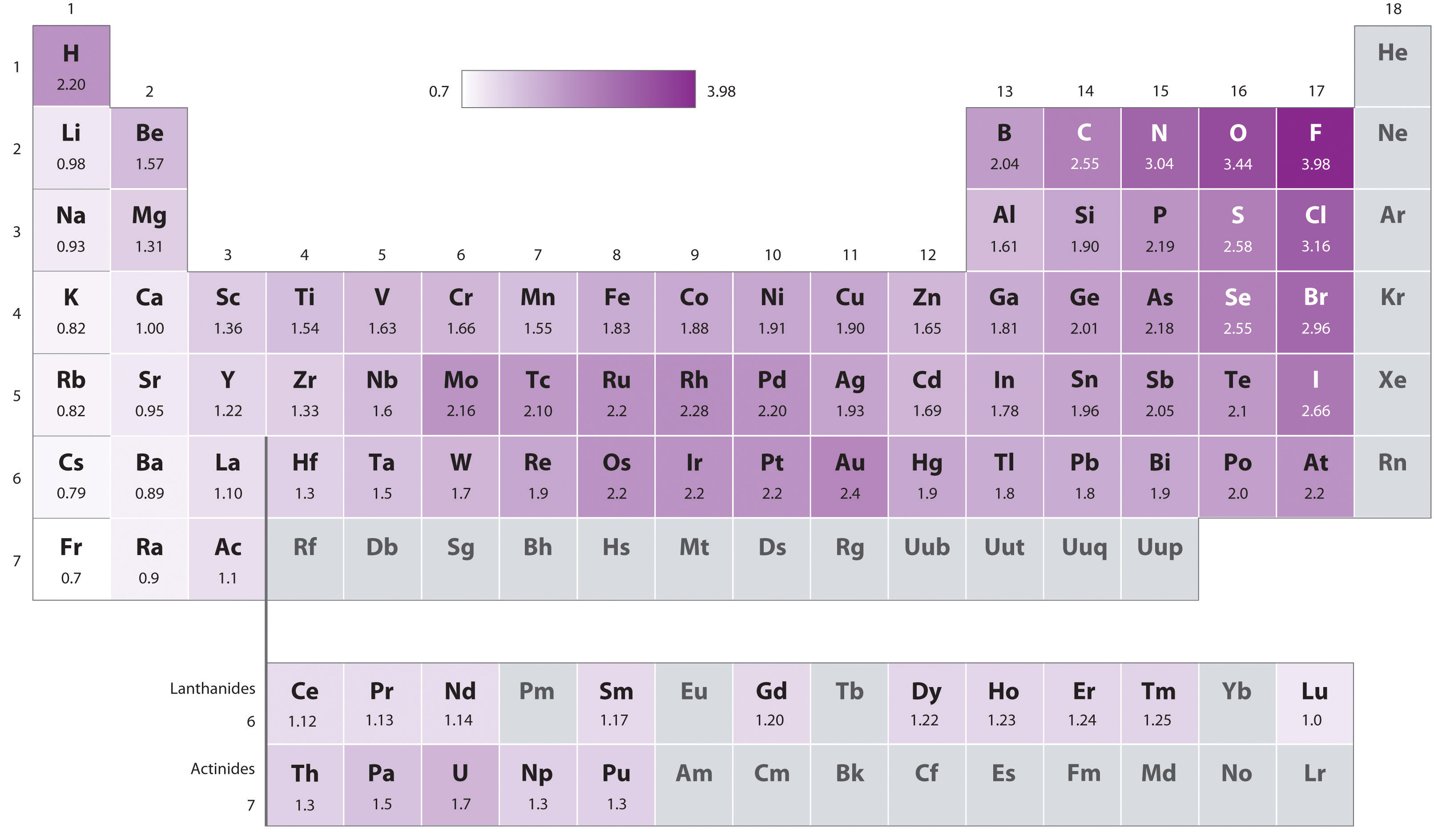
Electronegativity Bromine Difference . The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Three different conditions determine the type of chemical bond that the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. This page explains what electronegativity is, and how and why it varies around the periodic table. It looks at the way that electronegativity. It can also be used to predict if the resulting molecule will be polar. The suggested values are all taken from. The ability of an atom in a molecule to attract shared electrons is called electronegativity. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Across a period from left to right the electronegativity of atoms increases. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Electronegativity is a measure of an atom’s attraction for the electrons in a bond.
from chem.libretexts.org
The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if the resulting molecule will be polar. Three different conditions determine the type of chemical bond that the. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Across a period from left to right the electronegativity of atoms increases. The suggested values are all taken from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. This page explains what electronegativity is, and how and why it varies around the periodic table. It looks at the way that electronegativity.
8.4 Bond Polarity and Electronegativity Chemistry LibreTexts
Electronegativity Bromine Difference It can also be used to predict if the resulting molecule will be polar. This page explains what electronegativity is, and how and why it varies around the periodic table. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. It looks at the way that electronegativity. Three different conditions determine the type of chemical bond that the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. The ability of an atom in a molecule to attract shared electrons is called electronegativity. The suggested values are all taken from. Across a period from left to right the electronegativity of atoms increases. It can also be used to predict if the resulting molecule will be polar.
From mungfali.com
Atom Electronegativity Chart Electronegativity Bromine Difference It looks at the way that electronegativity. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Three different conditions determine the type of chemical bond that the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103. Electronegativity Bromine Difference.
From www.slideshare.net
Lecture5 123.101 Electronegativity Bromine Difference Electronegativity is a measure of an atom’s attraction for the electrons in a bond. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Across a period from left to right the electronegativity of atoms increases. The suggested values are all taken from. 119 rows electronegativity. Electronegativity Bromine Difference.
From www.youtube.com
Electronegativity Difference Example YouTube Electronegativity Bromine Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It looks at the way that electronegativity. It can also be used to predict if the resulting molecule will be polar. The ability of an atom in a molecule to attract shared electrons is called electronegativity. 103 rows electronegativity is not a uniquely. Electronegativity Bromine Difference.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart pauling scale Pauling electronegativity scale Electronegativity Bromine Difference Across a period from left to right the electronegativity of atoms increases. It looks at the way that electronegativity. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Three different conditions determine the type of chemical bond that. Electronegativity Bromine Difference.
From www.sliderbase.com
Periodic Table of Electronegativities Electronegativity Bromine Difference It looks at the way that electronegativity. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Subtract the two electronegativity values and you will have the electronegativity difference of the two. Electronegativity Bromine Difference.
From www.slideserve.com
PPT Electronegativity and Polarity PowerPoint Presentation, free Electronegativity Bromine Difference 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Three different conditions determine the type of chemical bond that the. The suggested values are all taken from. Subtract the two electronegativity values and you will have the electronegativity difference of. Electronegativity Bromine Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Bromine Difference This page explains what electronegativity is, and how and why it varies around the periodic table. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It looks at the way that electronegativity. The most used definition. Electronegativity Bromine Difference.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart pauling scale Pauling electronegativity scale Electronegativity Bromine Difference This page explains what electronegativity is, and how and why it varies around the periodic table. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Three different conditions determine the type of chemical bond that the. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Across a period from. Electronegativity Bromine Difference.
From thonggiocongnghiep.com
What Is The Electronegativity Difference Of Na And Br A Chemical Insight Electronegativity Bromine Difference Electronegativity is a measure of an atom’s attraction for the electrons in a bond. It can also be used to predict if the resulting molecule will be polar. It looks at the way that electronegativity. The suggested values are all taken from. Three different conditions determine the type of chemical bond that the. 103 rows electronegativity is not a uniquely. Electronegativity Bromine Difference.
From dreamstime.com
Electronegativity Periodic Table Stock Image Image 38153931 Electronegativity Bromine Difference It can also be used to predict if the resulting molecule will be polar. The suggested values are all taken from. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. This page explains what electronegativity is, and. Electronegativity Bromine Difference.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Electronegativity Bromine Difference Across a period from left to right the electronegativity of atoms increases. The ability of an atom in a molecule to attract shared electrons is called electronegativity. It looks at the way that electronegativity. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. 103 rows electronegativity is not a uniquely defined. Electronegativity Bromine Difference.
From mungfali.com
Electronegativity Difference Chart Electronegativity Bromine Difference Three different conditions determine the type of chemical bond that the. This page explains what electronegativity is, and how and why it varies around the periodic table. Across a period from left to right the electronegativity of atoms increases. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It looks at the. Electronegativity Bromine Difference.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electronegativity Bromine Difference The suggested values are all taken from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It looks at the way that electronegativity. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. This page explains what electronegativity is, and how and why it varies around the. Electronegativity Bromine Difference.
From www.slideserve.com
PPT Electronegativity and Polarity PowerPoint Presentation, free Electronegativity Bromine Difference The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. The ability of an atom in a molecule to attract shared electrons is called electronegativity. The most used definition of electronegativity is that an. Electronegativity Bromine Difference.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Bromine Difference The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. The ability of an atom in a molecule to attract shared electrons is called electronegativity. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. This page explains what electronegativity is, and. Electronegativity Bromine Difference.
From thonggiocongnghiep.com
What Is The Electronegativity Difference Of Na And Br A Chemical Insight Electronegativity Bromine Difference The suggested values are all taken from. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. It looks at the way that electronegativity. It can also be used to predict if the resulting molecule will be polar. This page explains what electronegativity is, and how and why it varies around the. Electronegativity Bromine Difference.
From www.coursehero.com
[Solved] 6. Calculate the difference in electronegativity (AEN) for the Electronegativity Bromine Difference This page explains what electronegativity is, and how and why it varies around the periodic table. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The ability of an atom in a molecule to attract shared electrons is called electronegativity. It looks at the way that electronegativity. The suggested values are all. Electronegativity Bromine Difference.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Electronegativity Bromine Difference Electronegativity is a measure of an atom’s attraction for the electrons in a bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if the resulting molecule will be polar. Across a period from left to right the electronegativity of atoms increases. This page explains what electronegativity. Electronegativity Bromine Difference.
From www.chemtopper.com
electronegativity and periodic trends Online Chemistry Tutor Electronegativity Bromine Difference The ability of an atom in a molecule to attract shared electrons is called electronegativity. The suggested values are all taken from. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. This page explains what electronegativity is,. Electronegativity Bromine Difference.
From studyafrikander.z13.web.core.windows.net
How To Determine Electronegativity Electronegativity Bromine Difference It can also be used to predict if the resulting molecule will be polar. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Three different conditions determine the type of chemical bond that the. Electronegativity is. Electronegativity Bromine Difference.
From chordcharts.z28.web.core.windows.net
pauling scale chart Electronegativity bond scale Electronegativity Bromine Difference Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. It looks at the way that electronegativity. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. This page explains what electronegativity is, and how and why it. Electronegativity Bromine Difference.
From slideplayer.com
Chemical Bonding. ppt download Electronegativity Bromine Difference Three different conditions determine the type of chemical bond that the. The ability of an atom in a molecule to attract shared electrons is called electronegativity. This page explains what electronegativity is, and how and why it varies around the periodic table. It looks at the way that electronegativity. Across a period from left to right the electronegativity of atoms. Electronegativity Bromine Difference.
From mungfali.com
Electronegativity Difference Chart Electronegativity Bromine Difference Across a period from left to right the electronegativity of atoms increases. This page explains what electronegativity is, and how and why it varies around the periodic table. It can also be used to predict if the resulting molecule will be polar. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity. Electronegativity Bromine Difference.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Electronegativity Bromine Difference It can also be used to predict if the resulting molecule will be polar. Across a period from left to right the electronegativity of atoms increases. The ability of an atom in a molecule to attract shared electrons is called electronegativity. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It looks. Electronegativity Bromine Difference.
From www.numerade.com
SOLVEDDetermine the electronegativity difference, the probable bonding Electronegativity Bromine Difference 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Across a period from left to right the electronegativity of atoms increases. It looks at the way that electronegativity. The suggested values are all taken from. This page explains what electronegativity. Electronegativity Bromine Difference.
From chem.libretexts.org
8.4 Bond Polarity and Electronegativity Chemistry LibreTexts Electronegativity Bromine Difference The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Across a period from left to right the electronegativity of atoms increases. It can also be used to. Electronegativity Bromine Difference.
From www.numerade.com
SOLVED Calculate the electronegativity difference for a bond between Electronegativity Bromine Difference The suggested values are all taken from. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. It looks at the way that electronegativity. Across a period from left to right the electronegativity of atoms increases. Subtract the two electronegativity values and you will have the. Electronegativity Bromine Difference.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Bromine Difference Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The suggested values are all taken from. Across a period from. Electronegativity Bromine Difference.
From mungfali.com
Electronegativity Trend Explained Electronegativity Bromine Difference Across a period from left to right the electronegativity of atoms increases. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Three different conditions determine the type of chemical bond that the. Electronegativity is a measure of an atom’s attraction for the electrons in a. Electronegativity Bromine Difference.
From mungfali.com
Electronegativity Difference Chart Electronegativity Bromine Difference The suggested values are all taken from. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. This page explains what electronegativity is, and how and why it varies around the periodic table. Three different conditions determine the type of chemical bond that the. It looks at the way that electronegativity. The. Electronegativity Bromine Difference.
From mungfali.com
Electronegativity Difference Chart Electronegativity Bromine Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It looks at the way that electronegativity. Three different conditions determine the type of chemical bond that the. This page explains what electronegativity is, and how and why it varies around the periodic table. The most used definition of electronegativity is that an. Electronegativity Bromine Difference.
From www.alamy.com
Bromine chemical element with first ionization energy, atomic mass and Electronegativity Bromine Difference The ability of an atom in a molecule to attract shared electrons is called electronegativity. Across a period from left to right the electronegativity of atoms increases. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. Electronegativity is a measure of an atom’s attraction for the electrons in a bond. Three. Electronegativity Bromine Difference.
From slideplayer.com
Chemical Bonding I CHEMISTRY 161 Chapter 9 Chemical Bonding I ppt Electronegativity Bromine Difference The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. It can also be used to predict if the resulting molecule will be polar. This page explains what electronegativity is, and how and why it varies around the periodic table. Subtract the two electronegativity values and. Electronegativity Bromine Difference.
From chem.libretexts.org
13.5 Electronegativity and Polarity Chemistry LibreTexts Electronegativity Bromine Difference The suggested values are all taken from. It can also be used to predict if the resulting molecule will be polar. Three different conditions determine the type of chemical bond that the. Across a period from left to right the electronegativity of atoms increases. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. This. Electronegativity Bromine Difference.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar Electronegativity Bromine Difference This page explains what electronegativity is, and how and why it varies around the periodic table. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms. It can. Electronegativity Bromine Difference.