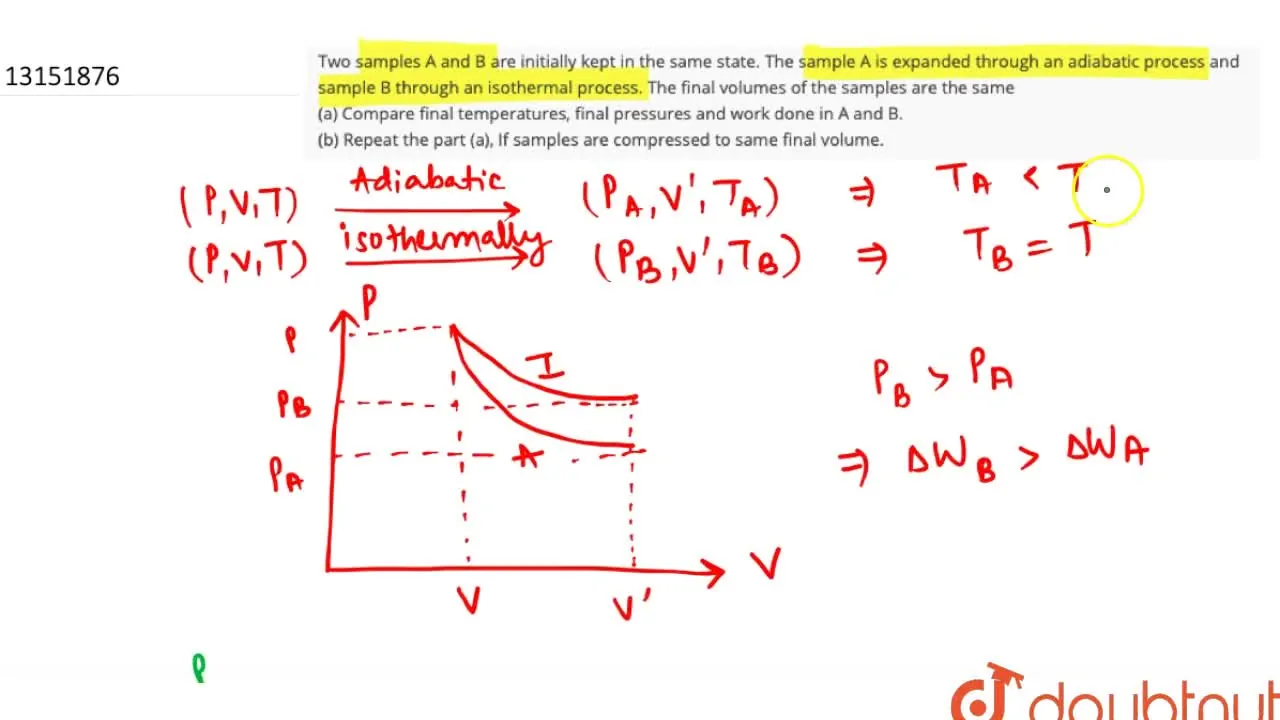
Two Samples A And B Are Initially Kept . Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. Two samples a and b are initially kept in the same state. A and b are then expanded to the same final volume through. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Initially, the temperatures of the two gases are equal. Two samples a and b are initially kept in the same state. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The sample a is expanded through an adiabatic process and sample b through an. The gases are electrically heated for some time during which equal amounts of heat are. Two different ideal diatomic gases a and b are initially in the same state. The sample a is expanded through an adiabatic process and the sample b through an. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. The sample a is expanded through an adiabatic process and the sample b.
from www.doubtnut.com
The sample a is expanded through an adiabatic process and the sample b through an. Two samples a and b are initially kept in the same state. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The sample a is expanded through an adiabatic process and sample b through an. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. Initially, the temperatures of the two gases are equal. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Two different ideal diatomic gases a and b are initially in the same state. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a.
Two samples A and B are initially kept in the same state. The sample B
Two Samples A And B Are Initially Kept Initially, the temperatures of the two gases are equal. The sample a is expanded through an adiabatic process and sample b through an. The sample a is expanded through an adiabatic process and the sample b. Two samples a and b are initially kept in the same state. Two samples a and b are initially kept in the same state. Initially, the temperatures of the two gases are equal. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. The sample a is expanded through an adiabatic process and the sample b through an. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The gases are electrically heated for some time during which equal amounts of heat are. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two different ideal diatomic gases a and b are initially in the same state. A and b are then expanded to the same final volume through. Two samples a and b are initially kept in the same state.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. The sample a is expanded through an adiabatic process and the sample b through an. A and b are then expanded to the same final volume through. Two sample a and b of a gas initially at the square at the same pressure and temperature. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. A and b are then expanded to the same final volume through. The sample a is expanded through an adiabatic process and sample b through an. Two samples a and b are initially kept in the same state. The sample $a$ is expanded through an adiabatic process and. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two sample A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. A and b are then expanded to the same final volume through. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two sample $a$ and $b$. Two Samples A And B Are Initially Kept.
From www.doubtnut.com
Two samples A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Two samples a and b are initially kept in the same state. Two different ideal diatomic gases a and b are initially in the same state. Two samples a and b are initially kept in the same state. Two sample a and b of a. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples of a gas A and B, initially same temperature and pressure Two Samples A And B Are Initially Kept Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Initially, the temperatures of the two gases are equal. Two samples a and b are initially kept. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two sample A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept The sample a is expanded through an adiabatic process and sample b through an. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The gases are electrically heated for some time during which equal amounts of heat are. A and b are then. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two sample A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept Initially, the temperatures of the two gases are equal. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b of a certain. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. Initially, the temperatures of the two gases are equal. The sample a is expanded through an adiabatic process and sample b. Two Samples A And B Are Initially Kept.
From www.doubtnut.com
[Marathi] Two samples A and B of a gas initially of the same temperatu Two Samples A And B Are Initially Kept Initially, the temperatures of the two gases are equal. The sample a is expanded through an adiabatic process and the sample b through an. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b are initially kept. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same pressure and Two Samples A And B Are Initially Kept The sample a is expanded through an adiabatic process and sample b through an. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The sample a is expanded through an adiabatic process and the sample b. A and b are then expanded to. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same pressure and Two Samples A And B Are Initially Kept Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. The gases are electrically heated for some time during which equal amounts of heat are. Two samples a and b are initially kept in the same state. The sample a is expanded. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two samples a and b are initially kept in the same state. Initially, the temperatures of the two gases are equal. The sample a is expanded through an adiabatic process and the sample b. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2.. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B are initially kept in the same state. Sample A is Two Samples A And B Are Initially Kept Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. Two different ideal diatomic gases a and b are initially in the same state. Two sample $a$ and $b$ are initially kept in the same state. Initially, the temperatures of the two gases are. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Initially, the temperatures of the two gases are equal. Two samples a and b are initially kept in the same state. A and b are then expanded to the same final volume through. The sample a is expanded through an adiabatic process and sample b through an. Two different ideal diatomic gases a and b are initially in the same. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. The gases are electrically heated for some time during which equal amounts of heat are. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Initially, the temperatures of the two gases are equal. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The gases are electrically heated for some time during which equal amounts of heat are. Two different ideal diatomic gases a and b are initially. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. A and b are then expanded to the same final volume through. The gases are electrically heated for some time during which equal amounts of heat are. The sample a is expanded through an adiabatic process and the sample b. Two samples a and b are initially kept. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same pressure and Two Samples A And B Are Initially Kept Two samples a and b are initially kept in the same state. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Initially, the temperatures of the two gases are equal. The sample $a$ is expanded through an adiabatic process and the. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples of a gas A and B, initially same temperature and pressure Two Samples A And B Are Initially Kept Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b are. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two samples a and b are initially kept in the same state. Initially, the temperatures of the two gases are equal. Two sample $a$ and $b$ are initially kept in the same state. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. The sample a is expanded through an adiabatic process and sample b. Two Samples A And B Are Initially Kept.
From www.toppr.com
81. Two samples A and B are initially kept in the same state. The Two Samples A And B Are Initially Kept The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. The sample a is expanded through an adiabatic process and the sample b. Two samples a and b are initially kept in the same state. The sample a is expanded through an adiabatic process and sample b through an. Two sample $a$ and $b$ are. Two Samples A And B Are Initially Kept.
From www.youtube.com
Two samples `1` and `2` are initially kept in the same state. The Two Samples A And B Are Initially Kept The gases are electrically heated for some time during which equal amounts of heat are. The sample a is expanded through an adiabatic process and the sample b. Initially, the temperatures of the two gases are equal. The sample a is expanded through an adiabatic process and sample b through an. Two samples a and b are initially kept in. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two samples a and b are initially kept in the same state. The sample a is expanded through an adiabatic process and the sample b. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two sample $a$ and $b$ are initially. Two Samples A And B Are Initially Kept.
From byjus.com
Two samples A and B initially at the same temperature and pressure and Two Samples A And B Are Initially Kept The sample a is expanded through an adiabatic process and the sample b through an. Initially, the temperatures of the two gases are equal. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two sample A and B ar process and (C) oxygen P consta ample A and B are Two Samples A And B Are Initially Kept The sample a is expanded through an adiabatic process and the sample b through an. Two sample $a$ and $b$ are initially kept in the same state. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Initially, the temperatures of the. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. The sample a is expanded through an adiabatic process and sample b through an. Initially, the temperatures of the two gases are equal. The gases are electrically heated for some time during which equal amounts of. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. Initially, the temperatures of the two gases are equal. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Two samples a and b are initially kept in the. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept A and b are then expanded to the same final volume through. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. The sample $a$ is expanded through an adiabatic process and the sample $b$ through an. Initially, the temperatures of the two gases are equal.. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. The sample a is expanded through. Two Samples A And B Are Initially Kept.
From www.doubtnut.com
Two samples A and B are initially kept in the same state. The sample B Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. Two different ideal diatomic gases a and b are initially in the same state. Two samples a and b are initially kept in the same state. The gases are electrically heated for some time during which equal amounts of heat are. Two samples a and b of a. Two Samples A And B Are Initially Kept.
From www.doubtnut.com
Two samples A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept The gases are electrically heated for some time during which equal amounts of heat are. The sample a is expanded through an adiabatic process and the sample b. Two samples a and b are initially kept in the same state. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed. Two Samples A And B Are Initially Kept.
From www.doubtnut.com
Two samples A and B are initially kept in the same state. The sample B Two Samples A And B Are Initially Kept Two sample $a$ and $b$ are initially kept in the same state. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed. Two Samples A And B Are Initially Kept.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Are Initially Kept Two samples a and b of a certain gas, which are initially at the same temperature and pressure, are compressed from volume v to v /2. Two sample a and b of a gas initially at the square at the same pressure and temperature are compressed from volume v to v / 2 (a. Two samples a and b are. Two Samples A And B Are Initially Kept.
From www.youtube.com
Two samples A and B are initially kept in the same state. The sample A Two Samples A And B Are Initially Kept The sample a is expanded through an adiabatic process and the sample b. Two different ideal diatomic gases a and b are initially in the same state. Two samples a and b are initially kept in the same state. Two samples a and b are initially kept in the same state. The sample $a$ is expanded through an adiabatic process. Two Samples A And B Are Initially Kept.
From www.youtube.com
Two samples `1` and `2` are initially kept in the same state. The Two Samples A And B Are Initially Kept Two different ideal diatomic gases a and b are initially in the same state. Two sample $a$ and $b$ are initially kept in the same state. Two samples a and b are initially kept in the same state. The sample a is expanded through an adiabatic process and sample b through an. The sample a is expanded through an adiabatic. Two Samples A And B Are Initially Kept.