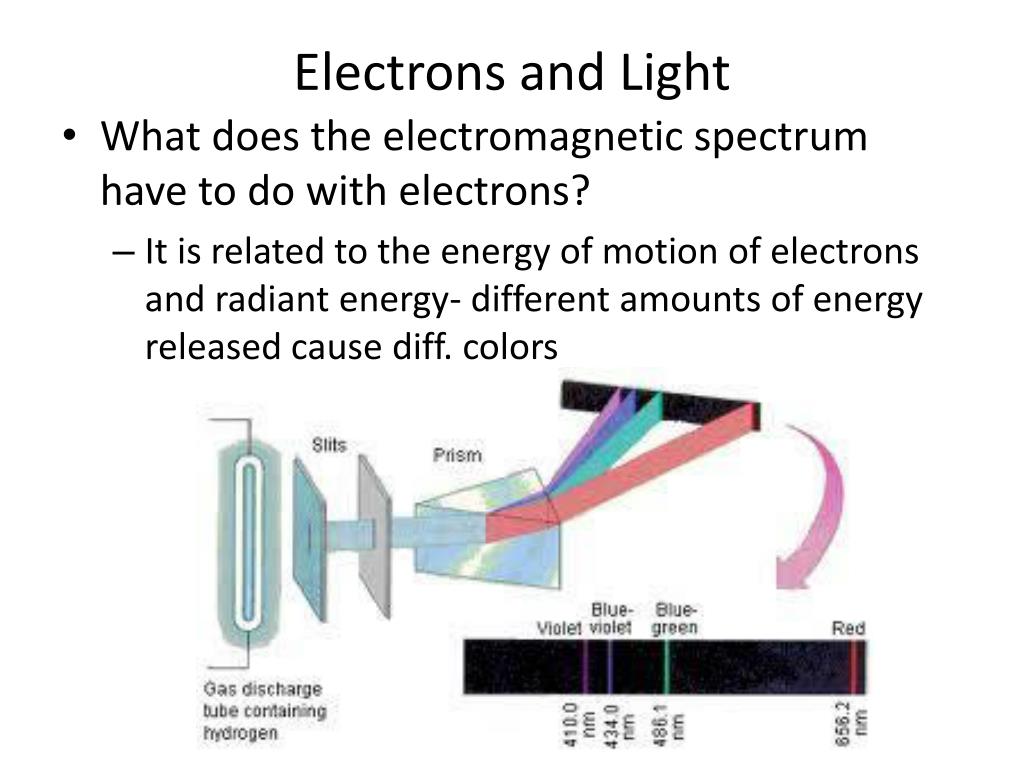
How Do Electrons Travel . Each collision results in the. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). But when we specifically talk about the movement of an electron around a nucleus, it. Bohr's explanation of line spectra. He suggested that electrons are particles and they undergo two kinds of motion in atoms; A calculation shows that the electron is traveling at about 2,200 kilometers per second. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. That's less than 1% of the speed of light, but it's fast. Bohr atom the electron travels in circular orbits around the nucleus. An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. If you flick a string, a traveling wave moves down it; According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. The orbits have quantized sizes and energies. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. They either move continuously around the nucleus in.
from www.slideserve.com
Bohr's explanation of line spectra. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. But when we specifically talk about the movement of an electron around a nucleus, it. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). A calculation shows that the electron is traveling at about 2,200 kilometers per second. The orbits have quantized sizes and energies. He suggested that electrons are particles and they undergo two kinds of motion in atoms; According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases.
PPT Electrons in Atoms PowerPoint Presentation, free download ID
How Do Electrons Travel Each collision results in the. An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. That's less than 1% of the speed of light, but it's fast. If you flick a string, a traveling wave moves down it; Bohr's explanation of line spectra. But when we specifically talk about the movement of an electron around a nucleus, it. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. Each collision results in the. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). A calculation shows that the electron is traveling at about 2,200 kilometers per second. He suggested that electrons are particles and they undergo two kinds of motion in atoms; Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. They either move continuously around the nucleus in. Bohr atom the electron travels in circular orbits around the nucleus.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID7005589 How Do Electrons Travel An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. They either move continuously around the nucleus in. Each collision results in the. But when we specifically talk about the movement of an electron around a nucleus, it. A calculation shows that the electron is. How Do Electrons Travel.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind How Do Electrons Travel Each collision results in the. That's less than 1% of the speed of light, but it's fast. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. A calculation shows that the electron is traveling at about 2,200 kilometers per. How Do Electrons Travel.
From www.slideserve.com
PPT KS4 Electricity Simple Circuits PowerPoint Presentation, free How Do Electrons Travel According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. That's less than 1% of the speed of light, but it's fast. Each collision results in the. If you flick a string, a traveling wave moves down it; Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). The orbits. How Do Electrons Travel.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID3014594 How Do Electrons Travel An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. If you flick a string, a traveling wave moves down it; They either move continuously around the nucleus in. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). He suggested that electrons are. How Do Electrons Travel.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Do Electrons Travel That's less than 1% of the speed of light, but it's fast. An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. Bohr's explanation of line spectra. But when we specifically talk about the movement of an electron around a nucleus, it. If you flick. How Do Electrons Travel.
From ibiologia.com
Electron Transport Chain Introduction , Steps & Examples How Do Electrons Travel Each collision results in the. A calculation shows that the electron is traveling at about 2,200 kilometers per second. Bohr atom the electron travels in circular orbits around the nucleus. Bohr's explanation of line spectra. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were. How Do Electrons Travel.
From microbenotes.com
Electron Transport Chain Steps, Products, Diagram How Do Electrons Travel But when we specifically talk about the movement of an electron around a nucleus, it. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). They either move continuously around the nucleus in. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. An electron's journey through a circuit can. How Do Electrons Travel.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Do Electrons Travel A calculation shows that the electron is traveling at about 2,200 kilometers per second. If you flick a string, a traveling wave moves down it; Bohr atom the electron travels in circular orbits around the nucleus. Bohr's explanation of line spectra. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which. How Do Electrons Travel.
From www.allaboutcircuits.com
Electrons and “holes’’ Solidstate Device Theory Electronics Textbook How Do Electrons Travel An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. Energy is emitted from the. How Do Electrons Travel.
From spmphysics.blog.onlinetuition.com.my
Cathode Ray SPM Physics How Do Electrons Travel If you flick a string, a traveling wave moves down it; More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. An electron's journey through a circuit can be described as a zigzag path that results from countless. How Do Electrons Travel.
From www.lifeschemistrypress.com
5. The reality of electrons Life's Chemistry Press How Do Electrons Travel The orbits have quantized sizes and energies. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. A calculation shows that the electron is traveling at about. How Do Electrons Travel.
From www.lihpao.com
How Fast Do Electrons Travel? Exploring the Physics Behind Electron How Do Electrons Travel They either move continuously around the nucleus in. Bohr atom the electron travels in circular orbits around the nucleus. The orbits have quantized sizes and energies. But when we specifically talk about the movement of an electron around a nucleus, it. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus.. How Do Electrons Travel.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Do Electrons Travel Bohr atom the electron travels in circular orbits around the nucleus. An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and. How Do Electrons Travel.
From www.tffn.net
How Fast Do Electrons Travel? Exploring the Physics Behind Electron How Do Electrons Travel In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. That's less than 1% of the speed of light, but it's fast. The orbits have quantized sizes and energies. More widely, from caesium $(z = 55)$ on up the inner. How Do Electrons Travel.
From www.slideserve.com
PPT Circuit Schematic Drawings PowerPoint Presentation, free download How Do Electrons Travel They either move continuously around the nucleus in. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. But. How Do Electrons Travel.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps How Do Electrons Travel That's less than 1% of the speed of light, but it's fast. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). Bohr's explanation of line spectra. Bohr atom the electron travels in circular orbits around the nucleus. If you. How Do Electrons Travel.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art How Do Electrons Travel Bohr's explanation of line spectra. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). A calculation shows that the electron is traveling at about 2,200 kilometers. How Do Electrons Travel.
From sciencenotes.org
Where Are the Electrons Located in an Atom? How Do Electrons Travel A calculation shows that the electron is traveling at about 2,200 kilometers per second. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. He suggested that electrons are particles and they undergo two kinds of motion in atoms; An electron's journey through a circuit can be described as a zigzag. How Do Electrons Travel.
From www.youtube.com
15. Do free electrons travel to region of higher potential or lower How Do Electrons Travel In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. A calculation shows that the electron is traveling at about 2,200 kilometers per second. They either move continuously around the nucleus in. The orbits have quantized sizes and energies. More. How Do Electrons Travel.
From slideplayer.com
Atoms General Science ppt download How Do Electrons Travel They either move continuously around the nucleus in. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. Each collision results in the. But when we specifically talk about the movement of an electron around a nucleus, it. Energy is. How Do Electrons Travel.
From www.slideserve.com
PPT The Building Blocks PowerPoint Presentation, free download ID How Do Electrons Travel Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Bohr's explanation of line spectra. Bohr atom the electron travels in circular orbits around the nucleus. That's less than 1% of the speed of light, but it's fast. According to the wave particle duality, an electron behaves like a wave and. How Do Electrons Travel.
From slideplayer.com
Chapter 4 September 21, ppt download How Do Electrons Travel According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. That's less than 1% of the speed of light, but it's fast. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half. How Do Electrons Travel.
From dokumen.tips
(PPT) Periodic Table. Bell Ringer What is this model of the atom called How Do Electrons Travel In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. Bohr atom the electron travels in circular orbits around the nucleus. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half. How Do Electrons Travel.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor How Do Electrons Travel More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce. How Do Electrons Travel.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript How Do Electrons Travel Each collision results in the. Bohr atom the electron travels in circular orbits around the nucleus. He suggested that electrons are particles and they undergo two kinds of motion in atoms; The orbits have quantized sizes and energies. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. According to the. How Do Electrons Travel.
From www.expii.com
Electron Transport Chain — Summary & Diagrams Expii How Do Electrons Travel He suggested that electrons are particles and they undergo two kinds of motion in atoms; An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons. How Do Electrons Travel.
From www.youtube.com
Why do electrons travel in fixed paths in Bohr's model? YouTube How Do Electrons Travel Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed of light, and from francium $(z=87)$ about half the electrons have. If you flick a string, a traveling wave moves down it; Bohr atom the electron travels in. How Do Electrons Travel.
From chemistry.stackexchange.com
physical chemistry How do electrons travel through nodes Chemistry How Do Electrons Travel An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. If you flick a string, a traveling wave moves down it; But when we specifically talk about the movement of an electron around a nucleus, it. According to the wave particle duality, an electron behaves. How Do Electrons Travel.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID How Do Electrons Travel Bohr's explanation of line spectra. That's less than 1% of the speed of light, but it's fast. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. The orbits have quantized sizes and energies. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). More widely, from caesium $(z =. How Do Electrons Travel.
From www.mathwizurd.com
Electron Transport Chain — Mathwizurd How Do Electrons Travel They either move continuously around the nucleus in. He suggested that electrons are particles and they undergo two kinds of motion in atoms; But when we specifically talk about the movement of an electron around a nucleus, it. That's less than 1% of the speed of light, but it's fast. Energy is emitted from the atom when the electron jumps. How Do Electrons Travel.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent How Do Electrons Travel He suggested that electrons are particles and they undergo two kinds of motion in atoms; A calculation shows that the electron is traveling at about 2,200 kilometers per second. That's less than 1% of the speed of light, but it's fast. If you flick a string, a traveling wave moves down it; Understanding bohr's model requires some knowledge of electromagnetic. How Do Electrons Travel.
From www.youtube.com
Electron Flow in Conductors YouTube How Do Electrons Travel Bohr atom the electron travels in circular orbits around the nucleus. An electron's journey through a circuit can be described as a zigzag path that results from countless collisions with the atoms of the conducting wire. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). The orbits have quantized sizes and energies. He suggested that electrons are particles. How Do Electrons Travel.
From www.slideserve.com
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID9583765 How Do Electrons Travel Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. They either move continuously around the nucleus in. Each collision results in the. The orbits have quantized sizes and energies. More widely, from caesium $(z = 55)$ on up the inner electrons have speeds of the order of half the speed. How Do Electrons Travel.
From slideplayer.com
Modern Theories of the Atom ppt download How Do Electrons Travel A calculation shows that the electron is traveling at about 2,200 kilometers per second. According to the wave particle duality, an electron behaves like a wave and as a particle in certain cases. Bohr atom the electron travels in circular orbits around the nucleus. Each collision results in the. The orbits have quantized sizes and energies. Energy is emitted from. How Do Electrons Travel.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Do Electrons Travel The orbits have quantized sizes and energies. If you flick a string, a traveling wave moves down it; Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. That's less than 1% of the speed of light, but it's fast.. How Do Electrons Travel.