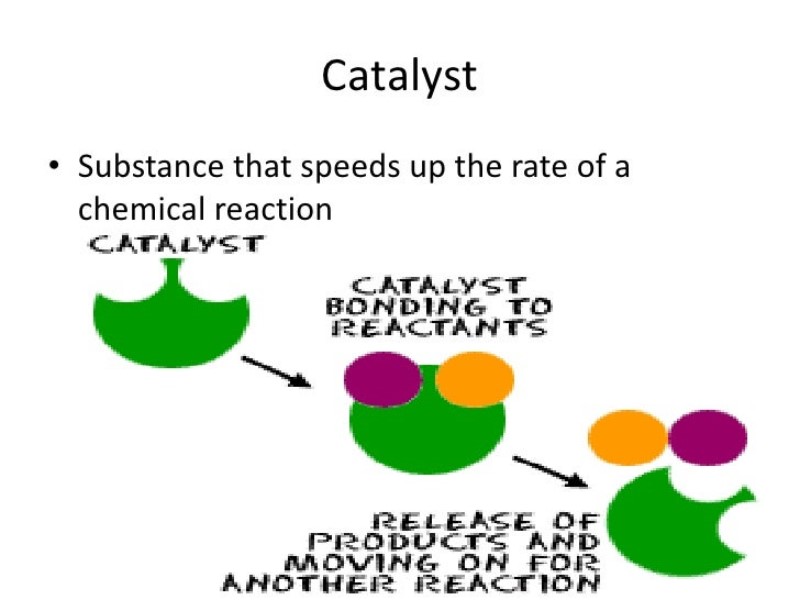
Catalyst Definition Bbc Bitesize . A catalyst is a substance that speeds up a chemical reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Different reactions require different catalysts. The catalyst does not take. However, not all reactions have suitable catalysts. A catalyst is a substance that will change the rate of a reaction. A catalyst is often used to make a reaction go faster. However, not all reactions have suitable catalysts. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Only a very small mass of catalyst is needed to increase the rate of a reaction.
from operaresidences.com.au
Catalysts are reaction specific, which means that we must use different catalysts for different reactions. A catalyst is a substance that will change the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. However, not all reactions have suitable catalysts. Different reactions require different catalysts. The catalyst does not take. A catalyst is a substance that speeds up a chemical reaction.
What does biological catalyst mean? Opera Residences
Catalyst Definition Bbc Bitesize There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Different reactions require different catalysts. A catalyst is often used to make a reaction go faster. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that speeds up a chemical reaction. The catalyst does not take. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst is a substance that will change the rate of a reaction.
From operaresidences.com.au
What does biological catalyst mean? Opera Residences Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different reactions require different catalysts. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a. Catalyst Definition Bbc Bitesize.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst is a substance that speeds up a chemical reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. However, not all reactions have suitable catalysts. There are also negative. Catalyst Definition Bbc Bitesize.
From www.pinterest.com
BBC GCSE Bitesize Biological catalysts Reaction Rate, Science Catalyst Definition Bbc Bitesize There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. The catalyst does not take. Usually when. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts guide for KS3 chemistry students BBC Bitesize Catalyst Definition Bbc Bitesize Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Different reactions require different catalysts. A catalyst is a substance that will change the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that speeds up a chemical reaction.. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts guide for KS3 chemistry students BBC Bitesize Catalyst Definition Bbc Bitesize Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. The catalyst does not take. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts are reaction specific, which means that we must. Catalyst Definition Bbc Bitesize.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Definition Bbc Bitesize A catalyst is a substance that speeds up a chemical reaction. The catalyst does not take. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. A catalyst is often used to make a reaction go faster. However, not all reactions have suitable catalysts. However, not all reactions have suitable catalysts. There are also negative. Catalyst Definition Bbc Bitesize.
From hxeiyngtd.blob.core.windows.net
Catalyst Definition Bbc at Veronica Howard blog Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate. Catalyst Definition Bbc Bitesize.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Definition Bbc Bitesize Catalysts are reaction specific, which means that we must use different catalysts for different reactions. A catalyst is a substance that speeds up a chemical reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. A catalyst is often. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalyst Definition Bbc Bitesize A catalyst is often used to make a reaction go faster. The catalyst does not take. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. Usually. Catalyst Definition Bbc Bitesize.
From www.youtube.com
Catalyst Definition of Catalyst with Example YouTube Catalyst Definition Bbc Bitesize A catalyst is often used to make a reaction go faster. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. However, not all reactions have suitable catalysts. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
BBC Two KS4 Curriculum Bites, Science 1416, Collision theory and how Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is often used to make a reaction go faster. Different reactions require different catalysts. However, not all reactions have suitable catalysts. A catalyst is a substance. Catalyst Definition Bbc Bitesize.
From www.scribd.com
Catalyst Definition PDF Catalysis Reaction Rate Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. A catalyst is a substance that will change the rate of a reaction. A catalyst is often used to make a reaction go faster. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. The catalyst does not take. A. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Definition Bbc Bitesize Different reactions require different catalysts. The catalyst does not take. A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone. Catalyst Definition Bbc Bitesize.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Definition Bbc Bitesize A catalyst is a substance that speeds up a chemical reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that will change the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different reactions require different. Catalyst Definition Bbc Bitesize.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. The catalyst does not take. Only a very small mass of catalyst is needed to increase the rate. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts guide for KS3 chemistry students BBC Bitesize Catalyst Definition Bbc Bitesize The catalyst does not take. However, not all reactions have suitable catalysts. A catalyst is a substance that speeds up a chemical reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Bbc Bitesize A catalyst is a substance that speeds up a chemical reaction. A catalyst is often used to make a reaction go faster. Different reactions require different catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Only a very small mass of catalyst is needed to increase the rate of a reaction. The catalyst. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts Chemical reactions KS3 Chemistry BBC Bitesize BBC Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different reactions require different catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. However,. Catalyst Definition Bbc Bitesize.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that speeds up a chemical reaction. Different reactions require different catalysts. A catalyst is a substance that will change the rate of a reaction. A catalyst is often used to make a reaction go faster. However, not all reactions. Catalyst Definition Bbc Bitesize.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Definition Bbc Bitesize A catalyst is a substance that will change the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. The catalyst does not take. There are also negative catalysts or inhibitors, which slow the rate. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Reaction Rate PowerPoint Presentation, free download ID3534068 Catalyst Definition Bbc Bitesize Catalysts are reaction specific, which means that we must use different catalysts for different reactions. However, not all reactions have suitable catalysts. Different reactions require different catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts guide for KS3 chemistry students BBC Bitesize Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. The catalyst does not take. A catalyst is often used to make a reaction go faster. A catalyst is a substance that speeds up a chemical reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. There are also. Catalyst Definition Bbc Bitesize.
From worksheetlistska.z21.web.core.windows.net
Enzyme Worksheets Answers What Is A Catalyst Catalyst Definition Bbc Bitesize A catalyst is often used to make a reaction go faster. A catalyst is a substance that speeds up a chemical reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Only. Catalyst Definition Bbc Bitesize.
From wordstodescribesomeone.com
catalyst definition catalyst meaning words to describe someone Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. A catalyst is a substance that will change the rate of a reaction. A catalyst is a substance that speeds up a chemical reaction. There are also negative catalysts or inhibitors, which slow the rate of a chemical. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts guide for KS3 chemistry students BBC Bitesize Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that will change the rate of a reaction. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. A catalyst is often used to make a reaction. Catalyst Definition Bbc Bitesize.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Different reactions require different catalysts. A catalyst is a substance that will change the rate of a reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Only a very small mass of catalyst is needed to increase. Catalyst Definition Bbc Bitesize.
From hxeiyngtd.blob.core.windows.net
Catalyst Definition Bbc at Veronica Howard blog Catalyst Definition Bbc Bitesize Different reactions require different catalysts. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the. Catalyst Definition Bbc Bitesize.
From www.bbc.co.uk
Catalysts Controlling the rate Higher Chemistry Revision BBC Bitesize Catalyst Definition Bbc Bitesize There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Only a very small mass of catalyst is. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Bbc Bitesize Different reactions require different catalysts. A catalyst is a substance that will change the rate of a reaction. A catalyst is often used to make a reaction go faster. However, not all reactions have suitable catalysts. A catalyst is a substance that speeds up a chemical reaction. The catalyst does not take. There are also negative catalysts or inhibitors, which. Catalyst Definition Bbc Bitesize.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalyst Definition Bbc Bitesize The catalyst does not take. A catalyst is often used to make a reaction go faster. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that will change the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a. Catalyst Definition Bbc Bitesize.
From hxeiyngtd.blob.core.windows.net
Catalyst Definition Bbc at Veronica Howard blog Catalyst Definition Bbc Bitesize There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. The catalyst does not take. A catalyst is often used to make a reaction go faster. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that. Catalyst Definition Bbc Bitesize.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Bbc Bitesize However, not all reactions have suitable catalysts. However, not all reactions have suitable catalysts. The catalyst does not take. Only a very small mass of catalyst is needed to increase the rate of a reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction. Catalyst Definition Bbc Bitesize.
From www.slideshare.net
Catalyst Catalyst Definition Bbc Bitesize A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance that will change the rate of a reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. The catalyst does not take. Usually when someone refers to a catalyst, they. Catalyst Definition Bbc Bitesize.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Definition Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Only a very small mass of catalyst is needed to increase the rate of a reaction. There are also negative catalysts or inhibitors, which slow the rate of a. Catalyst Definition Bbc Bitesize.