Sulfuric Acid Plus Lead Iv Oxide . Supplying energy to an external load discharges. It is used as the cathode of lead. The cell should then be charged for different lengths of time,. The reactions between metals and acids. Asked 5 years, 11 months ago. This page shows how the position of a metal in the reactivity series affects its reactions with. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. Theoretically, a lead storage battery. What should be the reaction between lead oxide and dilute sulfuric acid? The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage.
from www.youtube.com
The reactions between metals and acids. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The cell should then be charged for different lengths of time,. What should be the reaction between lead oxide and dilute sulfuric acid? The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). It is used as the cathode of lead. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. This page shows how the position of a metal in the reactivity series affects its reactions with. Theoretically, a lead storage battery. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte.
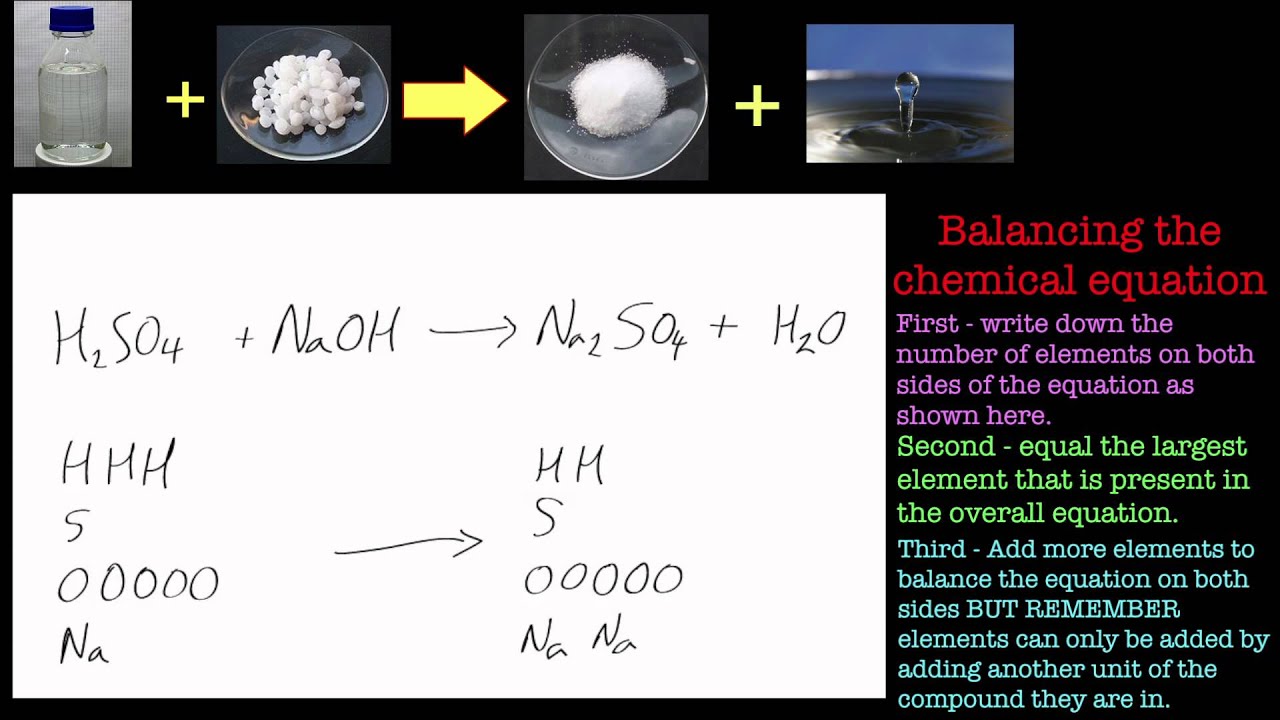
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium
Sulfuric Acid Plus Lead Iv Oxide What should be the reaction between lead oxide and dilute sulfuric acid? Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. The cell should then be charged for different lengths of time,. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). It is used as the cathode of lead. Asked 5 years, 11 months ago. The reactions between metals and acids. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. This page shows how the position of a metal in the reactivity series affects its reactions with. Theoretically, a lead storage battery. What should be the reaction between lead oxide and dilute sulfuric acid?
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Sulfuric Acid Plus Lead Iv Oxide The cell should then be charged for different lengths of time,. Supplying energy to an external load discharges. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reaction. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free Sulfuric Acid Plus Lead Iv Oxide Theoretically, a lead storage battery. This page shows how the position of a metal in the reactivity series affects its reactions with. Asked 5 years, 11 months ago. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reaction of lead and lead oxide with the sulfuric acid. Sulfuric Acid Plus Lead Iv Oxide.
From www.numerade.com
SOLVEDCar Battery Car batteries use lead, lead(IV) oxide, and a Sulfuric Acid Plus Lead Iv Oxide This page shows how the position of a metal in the reactivity series affects its reactions with. The cell should then be charged for different lengths of time,. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). What should be the reaction between lead oxide and dilute sulfuric. Sulfuric Acid Plus Lead Iv Oxide.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Sulfuric Acid Plus Lead Iv Oxide Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The cell should then be charged for different lengths of time,. Asked 5 years,. Sulfuric Acid Plus Lead Iv Oxide.
From www.thermofisher.com
Titanium(IV) oxide sulfate sulfuric acid hydrate, Thermo Scientific Sulfuric Acid Plus Lead Iv Oxide Theoretically, a lead storage battery. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery. Sulfuric Acid Plus Lead Iv Oxide.
From kunduz.com
[ANSWERED] The leadacid storage battery is the oldest rechargeable Sulfuric Acid Plus Lead Iv Oxide The reactions between metals and acids. Asked 5 years, 11 months ago. Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. This page shows how the position of a metal in the reactivity series affects its reactions with. The lead acid battery stores and. Sulfuric Acid Plus Lead Iv Oxide.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 2.4.6 Practical Investigate Metals Sulfuric Acid Plus Lead Iv Oxide The reactions between metals and acids. The cell should then be charged for different lengths of time,. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. Theoretically, a lead storage battery. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide,. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideserve.com
PPT The Lead Acid Electric Battery PowerPoint Presentation, free Sulfuric Acid Plus Lead Iv Oxide Asked 5 years, 11 months ago. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The reactions between metals and acids. Supplying energy to an external load discharges. Theoretically, a lead storage battery.. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideshare.net
Reaction types Sulfuric Acid Plus Lead Iv Oxide The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Theoretically, a lead storage battery. The cell should then be charged for different lengths of time,. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. Asked 5 years, 11 months ago. This reaction regenerates the. Sulfuric Acid Plus Lead Iv Oxide.
From exospsmtp.blob.core.windows.net
How Do Acids React With Bases at Clara Ryan blog Sulfuric Acid Plus Lead Iv Oxide Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. It is used as the cathode of lead. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead. Sulfuric Acid Plus Lead Iv Oxide.
From www.victoriana.com
Scharnier Innereien Bedienung möglich nitration mechanism verlorenes Sulfuric Acid Plus Lead Iv Oxide Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. This page shows how the position of a metal in the reactivity series affects its reactions with. The cell should then be charged for different lengths of time,. Asked 5 years, 11 months ago. The. Sulfuric Acid Plus Lead Iv Oxide.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Sulfuric Acid Plus Lead Iv Oxide It is used as the cathode of lead. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Theoretically, a lead storage battery. The lead acid battery stores and releases energy by shifting the equilibrium (a. Sulfuric Acid Plus Lead Iv Oxide.
From somaap.org
What is an aqueous acid in chemistry, 4 Reactions in Aqueous Solution Sulfuric Acid Plus Lead Iv Oxide Theoretically, a lead storage battery. Asked 5 years, 11 months ago. This page shows how the position of a metal in the reactivity series affects its reactions with. The cell should then be charged for different lengths of time,. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. What should be the reaction between. Sulfuric Acid Plus Lead Iv Oxide.
From www.youtube.com
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium Sulfuric Acid Plus Lead Iv Oxide What should be the reaction between lead oxide and dilute sulfuric acid? The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. Asked 5 years, 11 months ago. The reaction. Sulfuric Acid Plus Lead Iv Oxide.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Sulfuric Acid Plus Lead Iv Oxide It is used as the cathode of lead. The cell should then be charged for different lengths of time,. The reactions between metals and acids. This page shows how the position of a metal in the reactivity series affects its reactions with. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid. Sulfuric Acid Plus Lead Iv Oxide.
From classnotes.org.in
Sulphuric Acid Chemistry, Class 12, The pBlock Elements Sulfuric Acid Plus Lead Iv Oxide The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Supplying energy to an external load discharges. The reactions between metals and acids. The cell should then be charged for different lengths of time,. What should be the reaction between lead oxide and dilute sulfuric acid? Lead dioxide anodes were used for the production of. Sulfuric Acid Plus Lead Iv Oxide.
From dxouxxpeo.blob.core.windows.net
Do Bases React With All Metals at Virginia Albrecht blog Sulfuric Acid Plus Lead Iv Oxide This page shows how the position of a metal in the reactivity series affects its reactions with. What should be the reaction between lead oxide and dilute sulfuric acid? Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The reactions between metals and acids.. Sulfuric Acid Plus Lead Iv Oxide.
From daisy-yersblogchoi.blogspot.com
Word Equation for Copper Oxide and Sulfuric Acid Sulfuric Acid Plus Lead Iv Oxide Supplying energy to an external load discharges. This page shows how the position of a metal in the reactivity series affects its reactions with. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Theoretically, a lead storage battery. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideshare.net
Chemical Reactions Sulfuric Acid Plus Lead Iv Oxide What should be the reaction between lead oxide and dilute sulfuric acid? The cell should then be charged for different lengths of time,. Supplying energy to an external load discharges. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between. Sulfuric Acid Plus Lead Iv Oxide.
From klazoofqu.blob.core.windows.net
Zinc Hydroxide And Sulfuric Acid at Mamie Hunter blog Sulfuric Acid Plus Lead Iv Oxide The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reactions between metals and acids. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The cell should then be charged for different lengths of time,. It is used as. Sulfuric Acid Plus Lead Iv Oxide.
From ar.inspiredpencil.com
Sulfuric Acid And Water Sulfuric Acid Plus Lead Iv Oxide What should be the reaction between lead oxide and dilute sulfuric acid? The cell should then be charged for different lengths of time,. Supplying energy to an external load discharges. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. This page shows how the position of a metal in the reactivity series affects its. Sulfuric Acid Plus Lead Iv Oxide.
From klalixjsm.blob.core.windows.net
Copper Hydroxide Plus Sulfuric Acid at Mildred Anderson blog Sulfuric Acid Plus Lead Iv Oxide Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. Supplying energy to an external load discharges. It is used as the cathode of lead. Asked 5 years, 11 months ago. Theoretically, a lead storage battery. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between. Sulfuric Acid Plus Lead Iv Oxide.
From jaxkruwfletcher.blogspot.com
Copper Oxide Sulfuric Acid JaxkruwFletcher Sulfuric Acid Plus Lead Iv Oxide The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reactions between metals and acids. It is used as the cathode of lead. The cell should then be charged for different lengths of time,. This page shows how the position of a metal in the reactivity series affects. Sulfuric Acid Plus Lead Iv Oxide.
From www.youtube.com
How to Balance Pb(NO3)2 + H2SO4 = PbSO4 + HNO3 Lead (II) nitrate Sulfuric Acid Plus Lead Iv Oxide Asked 5 years, 11 months ago. Theoretically, a lead storage battery. What should be the reaction between lead oxide and dilute sulfuric acid? The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reactions between metals and acids. The cell should then be charged for different lengths of. Sulfuric Acid Plus Lead Iv Oxide.
From www.youtube.com
How to Balance H2S + O2 = H2O + SO2 (Hydrogen sulfide + Oxygen gas Sulfuric Acid Plus Lead Iv Oxide What should be the reaction between lead oxide and dilute sulfuric acid? Supplying energy to an external load discharges. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. It is used as the cathode of lead. Asked 5 years, 11 months ago. The cell should then be charged for different lengths. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideserve.com
PPT The Origins and Environmental Implications of Pollutants in Car Sulfuric Acid Plus Lead Iv Oxide This page shows how the position of a metal in the reactivity series affects its reactions with. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. Theoretically, a lead storage battery. Asked 5 years, 11 months. Sulfuric Acid Plus Lead Iv Oxide.
From slideplayer.com
Electrochemistry AP Chapter ppt download Sulfuric Acid Plus Lead Iv Oxide It is used as the cathode of lead. Theoretically, a lead storage battery. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The cell should then be charged for different. Sulfuric Acid Plus Lead Iv Oxide.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Sulfuric Acid Plus Lead Iv Oxide It is used as the cathode of lead. The cell should then be charged for different lengths of time,. This page shows how the position of a metal in the reactivity series affects its reactions with. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The reaction of lead and lead. Sulfuric Acid Plus Lead Iv Oxide.
From w20.b2m.cz
Mg Oh 2 H2so4 EDUCA Sulfuric Acid Plus Lead Iv Oxide The cell should then be charged for different lengths of time,. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Theoretically, a lead storage battery. What should be the reaction between lead oxide and dilute sulfuric acid? The reactions between metals and acids. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid. Sulfuric Acid Plus Lead Iv Oxide.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Sulfuric Acid Plus Lead Iv Oxide The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. The reactions between metals and acids. Theoretically, a lead storage battery. Asked 5 years, 11 months ago. The cell should. Sulfuric Acid Plus Lead Iv Oxide.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Sulfuric Acid Plus Lead Iv Oxide Theoretically, a lead storage battery. This page shows how the position of a metal in the reactivity series affects its reactions with. The reactions between metals and acids. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). The reaction of lead and lead oxide with the sulfuric acid. Sulfuric Acid Plus Lead Iv Oxide.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Sulfuric Acid Plus Lead Iv Oxide Supplying energy to an external load discharges. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Theoretically, a lead storage battery. The reactions between metals and acids. It is used as the cathode of lead. Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte.. Sulfuric Acid Plus Lead Iv Oxide.
From www.chegg.com
Solved A leadacid automotive battery consists of voltaic Sulfuric Acid Plus Lead Iv Oxide The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. Theoretically, a lead storage battery. Asked 5 years, 11 months ago. This reaction regenerates the lead, lead (iv) oxide, and. Sulfuric Acid Plus Lead Iv Oxide.
From www.dreamstime.com
Sulfurous Acid, Molecular Structures, Sulfuric Acid, 3d Model Sulfuric Acid Plus Lead Iv Oxide Supplying energy to an external load discharges. Theoretically, a lead storage battery. This reaction regenerates the lead, lead (iv) oxide, and sulfuric acid needed for the battery to function properly. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). This page shows how the position of a metal. Sulfuric Acid Plus Lead Iv Oxide.
From www.slideshare.net
Reaction Of Metal Oxides With Acid Sulfuric Acid Plus Lead Iv Oxide The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(ii). Asked 5 years, 11 months ago. What should be the reaction between lead oxide and dilute sulfuric acid? Lead dioxide anodes were used for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte. The cell. Sulfuric Acid Plus Lead Iv Oxide.