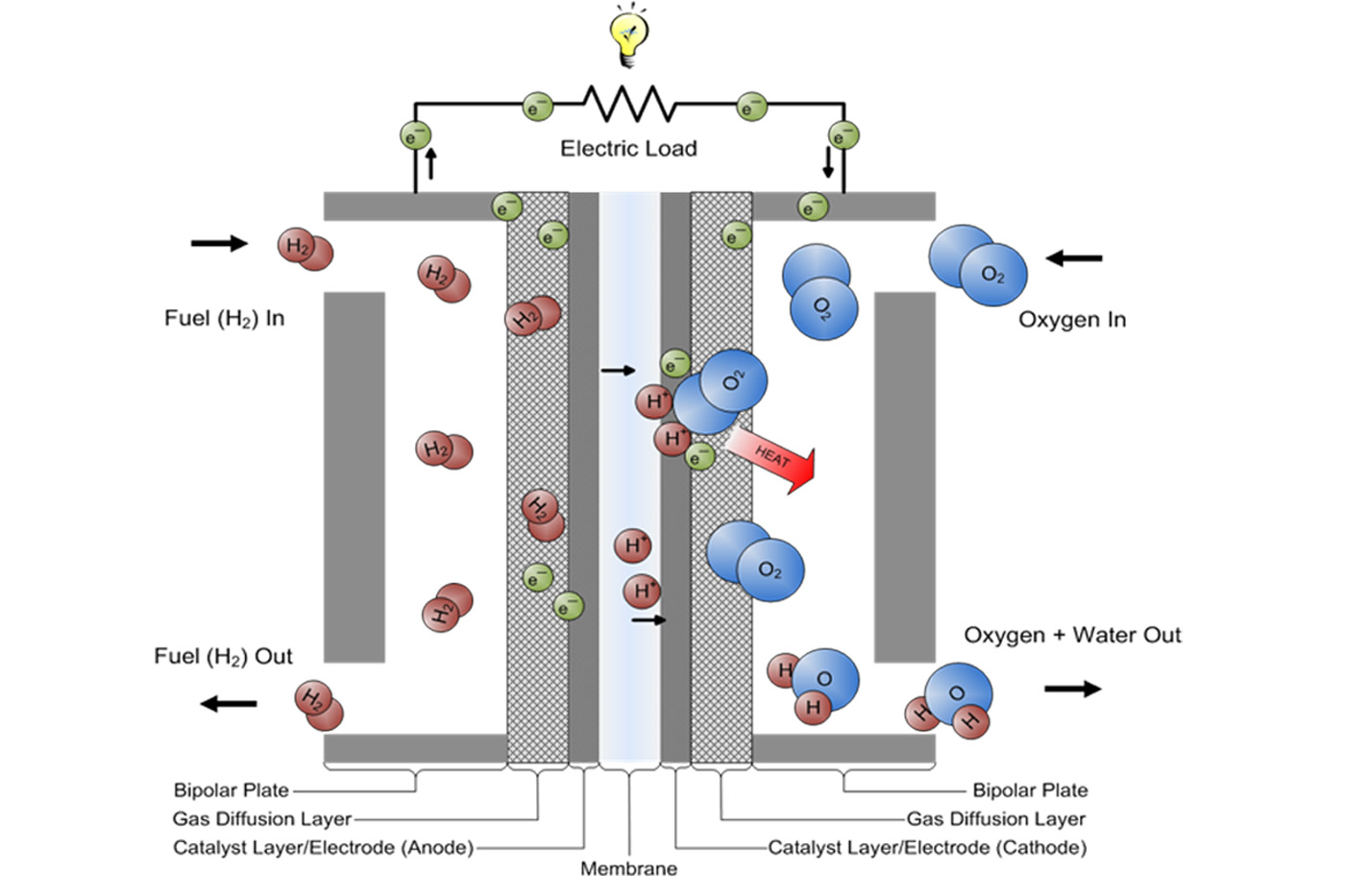
In H2O2 Fuel Cell Electrolyte Used Is . A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The correct option is c koh. Fuel cells have an important advantage. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst.
from www.change-florida.com
A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst.
Hydrogen Fuel Cells
In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the In H2O2 Fuel Cell Electrolyte Used Is The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a. In H2O2 Fuel Cell Electrolyte Used Is.
From www.semanticscholar.org
Figure 4 from A H2O2 fuel cell using acid doped polybenzimidazole as polymer electrolyte In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided. In H2O2 Fuel Cell Electrolyte Used Is.
From www.youtube.com
Trick to remember reactions of H2O2 Fuel cell/Electrochemistry/ASN CHEMISTRY YouTube In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From www.youtube.com
In `H_(2)O_(2)` fuel cell, the reaction occurring at cathode is YouTube In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. The general design of the h2−o2 fuel cell. In H2O2 Fuel Cell Electrolyte Used Is.
From www.doubtnut.com
Describe the construction and working of hydrogen oxygen (H(2) O(2) In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From brainly.in
Draw a neat labelled diagram of H2−O2 fuel cell. Write the reactions that occur at the cathode In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From www.slideserve.com
PPT Hydrogen peroxide fuel cell PowerPoint Presentation, free download ID3534590 In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle YouTube In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. Fuel cells have an important advantage. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From www.researchgate.net
3 A typical H 2 /O 2 PEM fuel cell. H 2 is oxidized at the anode... Download Scientific Diagram In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided. In H2O2 Fuel Cell Electrolyte Used Is.
From www.researchgate.net
Polymerelectrolytefuelcell schematic showing the reactantgas and... Download Scientific In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. Fuel cells have an important advantage. The correct option is c koh. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.denora.com
Anodes and Cathodes for Fuel Cells De Nora Electrode and Water Technologies that work! In H2O2 Fuel Cell Electrolyte Used Is The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided. In H2O2 Fuel Cell Electrolyte Used Is.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From electricala2z.com
Fuel Cell Types & Working PEMFC, SOFC, MCFC, PAFC, AFC Fuel Cell Electrical A2Z In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.youtube.com
Trick for H2 O2 Fuel cells YouTube In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce electrical energy as long as a. In H2O2 Fuel Cell Electrolyte Used Is.
From www.energy.gov
Hydrogen Production Electrolysis Department of Energy In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the reactions that occur at the In H2O2 Fuel Cell Electrolyte Used Is The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.semanticscholar.org
Figure 1 from A H2O2 fuel cell using acid doped polybenzimidazole as polymer electrolyte In H2O2 Fuel Cell Electrolyte Used Is The correct option is c koh. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.science.org
Direct electrosynthesis of pure aqueous H2O2 solutions up to 20 by weight using a solid In H2O2 Fuel Cell Electrolyte Used Is The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.researchgate.net
Operation of alkaline fuel cell; the electrolyte used in this system is... Download Scientific In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general. In H2O2 Fuel Cell Electrolyte Used Is.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.betase.nl
Electrochemistry of the fuel cell Betase BV In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. Fuel cells have an important advantage. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From semiengineering.com
Fuel Cells And The IoE In H2O2 Fuel Cell Electrolyte Used Is 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. In H2O2 Fuel Cell Electrolyte Used Is.
From mavink.com
Solid Fuel Cell Schematic In H2O2 Fuel Cell Electrolyte Used Is The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The correct option is c koh. Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working In H2O2 Fuel Cell Electrolyte Used Is The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. 1) the anode and cathode are porous carbon rods containing small amount of finely divided. In H2O2 Fuel Cell Electrolyte Used Is.
From www.elizabethqueenseaswann.com
PEROXIDE HYDROGEN H2O2 FUEL CELLS TANKS In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.fuelcellstore.com
Fuel Cell Electrolyte Layer Modeling In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell. In H2O2 Fuel Cell Electrolyte Used Is.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce electrical energy as long as a. In H2O2 Fuel Cell Electrolyte Used Is.
From www.studypool.com
SOLUTION Industrial chemistry h2o2 fuel cell with alkaline electrolyte Studypool In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. The correct option is c koh. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. A fuel cell like this will continue to operate and produce. In H2O2 Fuel Cell Electrolyte Used Is.
From www.cell.com
Electrochemical Synthesis of H2O2 by TwoElectron Water Oxidation Reaction Chem In H2O2 Fuel Cell Electrolyte Used Is The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.change-florida.com
Hydrogen Fuel Cells In H2O2 Fuel Cell Electrolyte Used Is A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.
From www.chfca.ca
About Fuel Cells CHFCA In H2O2 Fuel Cell Electrolyte Used Is The correct option is c koh. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a. In H2O2 Fuel Cell Electrolyte Used Is.
From slideplayer.com
Production of Liquid Solar Fuels and Their Use in Fuel Cells ppt download In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. 1) the anode and cathode are porous carbon rods containing small amount of finely divided platinum metal that acts as a catalyst. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell. In H2O2 Fuel Cell Electrolyte Used Is.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK In H2O2 Fuel Cell Electrolyte Used Is Fuel cells have an important advantage. The correct option is c koh. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The general design of the h2−o2 fuel cell consist of porous carbon electrodes containing suitable. 1) the anode and cathode are porous carbon rods. In H2O2 Fuel Cell Electrolyte Used Is.