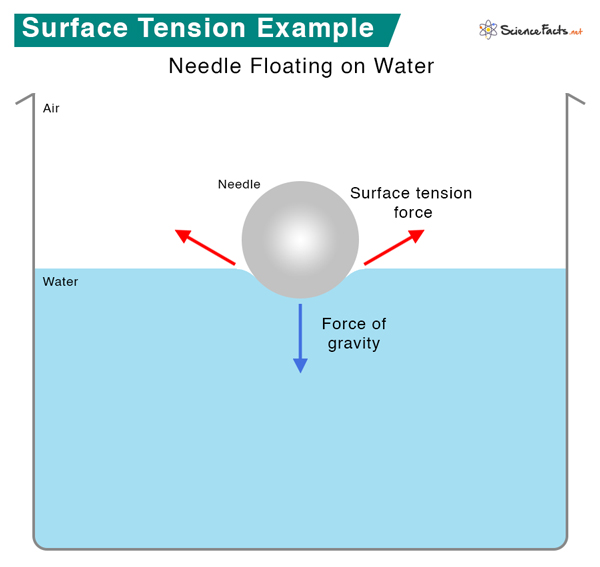
Surface Tension Higher Boiling Point . Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. For the analysis of the surface tension effects. Having defined vapor pressure, we can also redefine the boiling point of a liquid: The temperature at which the vapor pressure of a liquid equals the surrounding. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. One of the effects of intermolecular forces is surface tension. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid.
from www.sciencefacts.net
In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Having defined vapor pressure, we can also redefine the boiling point of a liquid: For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. One of the effects of intermolecular forces is surface tension. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. The temperature at which the vapor pressure of a liquid equals the surrounding. For the analysis of the surface tension effects. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule).
Surface Tension Definition, Examples, and Unit
Surface Tension Higher Boiling Point For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Having defined vapor pressure, we can also redefine the boiling point of a liquid: One of the effects of intermolecular forces is surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. For the analysis of the surface tension effects. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. The temperature at which the vapor pressure of a liquid equals the surrounding.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). One of the effects of intermolecular forces is surface tension. For the analysis of the surface tension effects. Having defined vapor pressure, we can also redefine the boiling point of a liquid: For example, water, with its strong intermolecular hydrogen bonding, has one of. Surface Tension Higher Boiling Point.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity, Surface Tension, Vapor Surface Tension Higher Boiling Point Having defined vapor pressure, we can also redefine the boiling point of a liquid: Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. In the absence of other forces, the surface tension makes. Surface Tension Higher Boiling Point.
From www.youtube.com
Experiment 16 Boiling Point, Surface Tension, and Space Boats, Part 2 YouTube Surface Tension Higher Boiling Point Having defined vapor pressure, we can also redefine the boiling point of a liquid: The temperature at which the vapor pressure of a liquid equals the surrounding. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension surface tension results from the net inward. Surface Tension Higher Boiling Point.
From sites.google.com
Raven Research Effects of Temperature and Salt on Surface Tension Surface Tension Higher Boiling Point Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. For the analysis of the surface tension effects. The temperature at which the vapor pressure of. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID4365473 Surface Tension Higher Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Having defined vapor pressure, we can also redefine the boiling point of a liquid: One of the effects of intermolecular forces is surface tension. For example, water, with its strong intermolecular hydrogen bonding, has one of. Surface Tension Higher Boiling Point.
From slideplayer.com
Intermolecular forces ppt download Surface Tension Higher Boiling Point The temperature at which the vapor pressure of a liquid equals the surrounding. Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). For the analysis of the surface tension effects. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. In the absence. Surface Tension Higher Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Surface Tension Higher Boiling Point In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. The temperature at which the vapor pressure of a liquid equals the surrounding. Having defined vapor pressure, we can also redefine the boiling point of a liquid: For the analysis of the surface tension effects. Surface. Surface Tension Higher Boiling Point.
From www.youtube.com
The surface tension of liquid at its boiling point YouTube Surface Tension Higher Boiling Point One of the effects of intermolecular forces is surface tension. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. For the analysis of the surface tension effects. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of. Surface Tension Higher Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Surface Tension Higher Boiling Point In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. For the analysis of the surface tension effects. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. The temperature at which the vapor pressure of. Surface Tension Higher Boiling Point.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition, Conditions, Characteristics Surface Tension Higher Boiling Point Having defined vapor pressure, we can also redefine the boiling point of a liquid: For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. In the. Surface Tension Higher Boiling Point.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Surface Tension Higher Boiling Point The temperature at which the vapor pressure of a liquid equals the surrounding. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Presentation ID6049698 Surface Tension Higher Boiling Point For the analysis of the surface tension effects. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Intermolecular force has a. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID2170124 Surface Tension Higher Boiling Point For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. For the analysis of the surface tension effects. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Surface tension is the elastic property of the surface of a liquid,. Surface Tension Higher Boiling Point.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Surface Tension Higher Boiling Point For the analysis of the surface tension effects. Having defined vapor pressure, we can also redefine the boiling point of a liquid: The temperature at which the vapor pressure of a liquid equals the surrounding. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Intermolecular force has a higher. Surface Tension Higher Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Surface Tension Higher Boiling Point The temperature at which the vapor pressure of a liquid equals the surrounding. For the analysis of the surface tension effects. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface. Surface Tension Higher Boiling Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Surface Tension Higher Boiling Point Having defined vapor pressure, we can also redefine the boiling point of a liquid: Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). The temperature at which the vapor pressure of a liquid equals the surrounding. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its. Surface Tension Higher Boiling Point.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Transcript Surface Tension Higher Boiling Point For the analysis of the surface tension effects. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Intermolecular force has a higher boiling point (look. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. One of the effects of intermolecular forces is surface tension. For the analysis of the surface tension effects. Surface tension surface tension results from. Surface Tension Higher Boiling Point.
From civilmint.com
What is Surface Tension Definition of Surface Tension, Examples and Test Surface Tension Higher Boiling Point In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. The temperature at which the vapor pressure of a liquid equals the surrounding. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Surface tension surface. Surface Tension Higher Boiling Point.
From slideplayer.com
Property Stronger forces mean… Viscosity Surface tension ppt download Surface Tension Higher Boiling Point The temperature at which the vapor pressure of a liquid equals the surrounding. One of the effects of intermolecular forces is surface tension. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Intermolecular force has a higher boiling point (look for functional groups that may. Surface Tension Higher Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point Practice Problems Chemistry Steps Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). For the analysis of the surface tension effects. The temperature at which the vapor pressure of a liquid equals the surrounding. Having defined vapor pressure, we can also redefine the boiling point of a liquid: In the absence of other forces, the surface tension. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 Surface Tension Higher Boiling Point For the analysis of the surface tension effects. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Intermolecular force has a higher boiling point (look for functional groups that. Surface Tension Higher Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube Surface Tension Higher Boiling Point Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\). Surface Tension Higher Boiling Point.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Surface Tension Higher Boiling Point For the analysis of the surface tension effects. The temperature at which the vapor pressure of a liquid equals the surrounding. Having defined vapor pressure, we can also redefine the boiling point of a liquid: For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Intermolecular force has a higher. Surface Tension Higher Boiling Point.
From www.youtube.com
Rank boiling points of alkanes [SMITH 5th] ORGANIC CHEMISTRY YouTube Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The temperature at which the vapor pressure of a liquid equals the surrounding. For the analysis of the surface tension. Surface Tension Higher Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). For the analysis of the surface tension effects. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The temperature at which the vapor pressure of a liquid equals the. Surface Tension Higher Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Surface Tension Higher Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The temperature at which the vapor pressure of a liquid equals the surrounding. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. For example, water,. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free download ID260245 Surface Tension Higher Boiling Point For the analysis of the surface tension effects. One of the effects of intermolecular forces is surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. The temperature at which the vapor pressure of a liquid equals the surrounding. In the absence of other forces, the surface tension. Surface Tension Higher Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Surface Tension Higher Boiling Point Having defined vapor pressure, we can also redefine the boiling point of a liquid: Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. One of the effects of intermolecular. Surface Tension Higher Boiling Point.
From www.youtube.com
Higher boiling point practice General Chemistry 2 Chapter 12 YouTube Surface Tension Higher Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. The temperature at which the vapor pressure of a liquid equals the surrounding. For the analysis. Surface Tension Higher Boiling Point.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). The temperature at which the vapor pressure of a liquid equals the surrounding. Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Surface tension is the energy required to increase the surface area. Surface Tension Higher Boiling Point.
From www.youtube.com
Experiment 16 Boiling Point, Surface Tension, and Space Boats, Part 1 YouTube Surface Tension Higher Boiling Point For the analysis of the surface tension effects. Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is the elastic property of the surface of a liquid,. Surface Tension Higher Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Surface Tension Higher Boiling Point Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. The temperature at which the vapor pressure of a liquid equals the surrounding. Having defined vapor pressure, we can also redefine the boiling point. Surface Tension Higher Boiling Point.
From www.numerade.com
SOLVEDPredict which liquid in each pair has the higher surface tension, higher viscosity, and Surface Tension Higher Boiling Point Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical. Having defined vapor pressure, we can also redefine the boiling point of a liquid: For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. In the absence of other forces,. Surface Tension Higher Boiling Point.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension Higher Boiling Point One of the effects of intermolecular forces is surface tension. Intermolecular force has a higher boiling point (look for functional groups that may indicate polar molecule). Surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest. Surface Tension Higher Boiling Point.