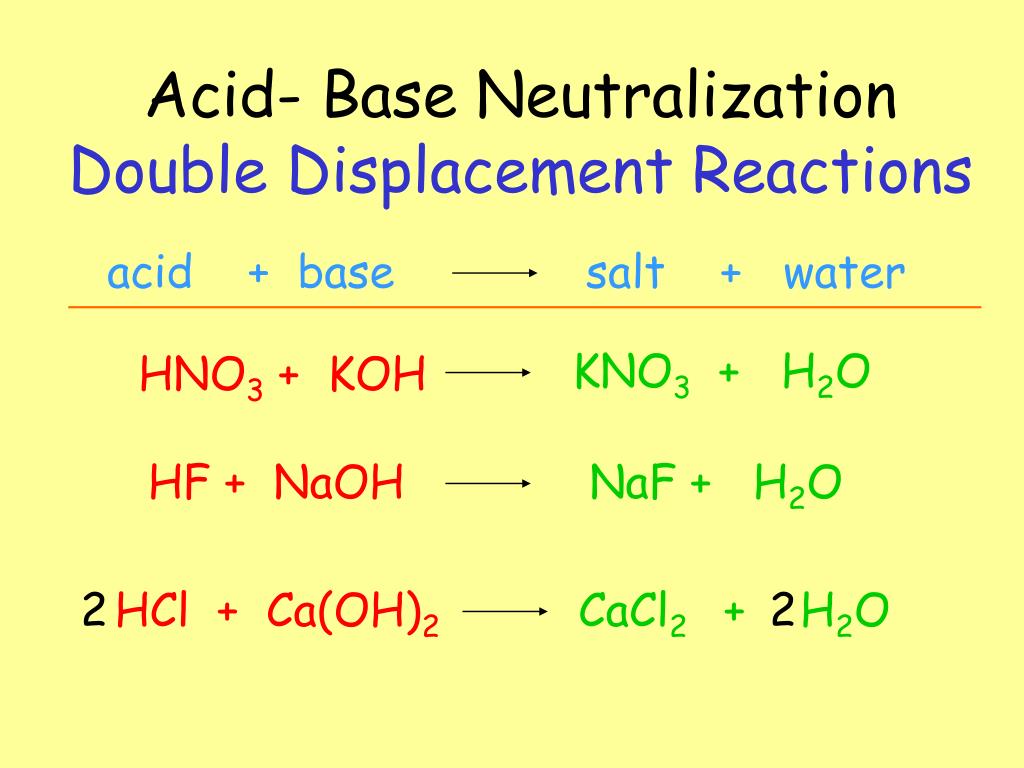
How To Neutralize Acidic Lakes Using An Acid-Base Reaction . When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. Because the acid has two h +. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. The general reaction is as follows:
from www.slideserve.com
H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. Because the acid has two h +. The general reaction is as follows: write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between.
PPT AcidBase Neutralization PowerPoint Presentation, free download
How To Neutralize Acidic Lakes Using An Acid-Base Reaction When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. Because the acid has two h +. The general reaction is as follows: H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. the reaction of an acid with a base is called a neutralisation reaction. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions.
From www.vrogue.co
Acid Base Equilibrium Part 1 How To Use The Pka Table vrogue.co How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. the reaction of an acid with a base is called. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.youtube.com
Adding a base to an acid How to neutralize acids YouTube How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction can. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From thechemistrynotes.com
AcidBase Reaction (Neutralization Reaction) How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. Because the acid has two h. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.masterorganicchemistry.com
Walkthrough of Acid Base Reactions (2) Basicity Master Organic Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic,. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From lessonlibrarypushups.z21.web.core.windows.net
Identify The Neutralization Reaction How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. a neutralization reaction is when an acid and a. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.masterorganicchemistry.com
Introduction to AcidBase Reactions Master Organic Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. the reaction of an acid with a base is called a neutralisation reaction. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From worksheetlistare.z21.web.core.windows.net
The Equation Shows A Neutralization Reaction How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. The general reaction is as follows: Because the acid has two h +. H 2 so 4. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.numerade.com
SOLVEDUsing chemical equations, explain how acid rain is neutralized How To Neutralize Acidic Lakes Using An Acid-Base Reaction When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. Because the acid has two h +. write balanced chemical equations for neutralization reactions and determine if the. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.youtube.com
Acid Base Neutralization Reactions Chemistry YouTube How To Neutralize Acidic Lakes Using An Acid-Base Reaction the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From answercampusbaecker.z13.web.core.windows.net
Chemistry Acid Base Reactions How To Neutralize Acidic Lakes Using An Acid-Base Reaction the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. Because. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.teachoo.com
MCQ What happens when a solution of an acid is mixed with a solution How To Neutralize Acidic Lakes Using An Acid-Base Reaction the reaction of an acid with a base is called a neutralisation reaction. The general reaction is as follows: a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. a neutralization reaction can be defined as a chemical reaction in which an. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Because the acid has. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.youtube.com
Writing balanced equations for acidbase neutralization reactions YouTube How To Neutralize Acidic Lakes Using An Acid-Base Reaction write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. Because the acid has two h +. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. The general reaction is as follows:. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.cleanwaterstore.com
How to Neutralize Acidic Water Clean Water Store Inc. How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. Because the acid has two h +. The general reaction is as follows:. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation ID358600 How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. write balanced chemical equations for neutralization reactions and determine if the resulting. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From sciencenotes.org
AcidBase Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. Because the acid has two h +. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. H 2 so 4 (aq) + koh (aq) → h 2 o. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From slideplayer.com
Acids and Bases pH factor. ppt download How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. The general reaction is as follows: write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. a neutralization reaction can be defined as a. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.chemistrysteps.com
Organic Acids and Bases Chemistry Steps How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. the reaction of an acid with a base is called a neutralisation reaction. Because the acid has two h +. When an acid (ph 7),. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.goodscience.com.au
Acids, Bases and pH Good Science How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. a neutralization reaction is when an acid and a base react to. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From study.com
How to Identify an AcidBase Reaction Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. Because the acid has two h +. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. The general reaction is as follows: a neutralization reaction can be. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. Because the acid has two h +. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. The general reaction is as follows:. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.slideserve.com
PPT Acid Lake Remediation PowerPoint Presentation, free download ID How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. H 2 so 4 (aq) + koh (aq). How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From printablelistmcguire.z22.web.core.windows.net
Chemical Reaction In Acidbase Neutralization How To Neutralize Acidic Lakes Using An Acid-Base Reaction write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions.. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. the reaction of an acid with a base is called a neutralisation reaction. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic,. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From morioh.com
Acids and Bases Basic Introduction Chemistry How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. The general reaction is as follows: the reaction of an acid with a base is called a neutralisation reaction. write balanced chemical equations. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.alamy.com
Acidbase reaction. chemical reaction neutralization the acid and base How To Neutralize Acidic Lakes Using An Acid-Base Reaction the reaction of an acid with a base is called a neutralisation reaction. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction can. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From slideplayer.com
Acids and Bases Science ppt download How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. Because the acid has two h +. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. write balanced chemical equations for neutralization reactions and determine if the resulting. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. the reaction of an acid with a base is called a neutralisation reaction. The general reaction is as follows: a neutralization reaction is. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From learningdbmulattoes.z13.web.core.windows.net
How To Do Acid Base Calculations How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions.. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.youtube.com
Neutralizing Acids and Bases YouTube How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. Because the acid has two h +. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. the reaction of an acid with a base is called a. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From slideplayer.com
Reactions in Aqueous Solutions ppt download How To Neutralize Acidic Lakes Using An Acid-Base Reaction The general reaction is as follows: H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Because the acid has two h +. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.slideserve.com
PPT Neutralization PowerPoint Presentation, free download ID1994775 How To Neutralize Acidic Lakes Using An Acid-Base Reaction a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between.. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.goodscience.com.au
AcidBase Reactions Good Science How To Neutralize Acidic Lakes Using An Acid-Base Reaction Because the acid has two h +. When an acid (ph 7), the ph of the resulting mixture will lie somewhere between. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. the reaction of an acid with a base is called a. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.
From www.slideserve.com
PPT AcidBase Neutralization PowerPoint Presentation, free download How To Neutralize Acidic Lakes Using An Acid-Base Reaction H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. a neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of h + ions. write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or.. How To Neutralize Acidic Lakes Using An Acid-Base Reaction.