Error Definition For Chemistry . It naturally results from the instruments we use, the way we use them, and factors outside our control. error is not an accident or mistake. errors in chemical analysis are simply defined as the difference between a measured value and the true value. sampling errors arise when a collected sample does not represent the environment being sampled. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. What is the difference between random and systematic error? revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams.
from thechemistrynotes.com
Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. error is not an accident or mistake. It naturally results from the instruments we use, the way we use them, and factors outside our control. sampling errors arise when a collected sample does not represent the environment being sampled. errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. What is the difference between random and systematic error?
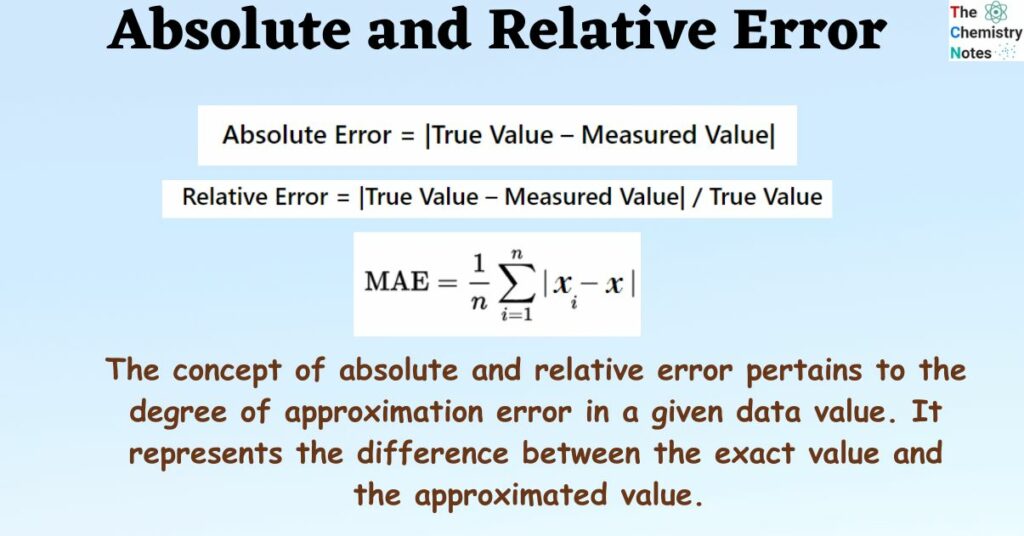
Absolute and Relative Error Definition, Formula, Examples, Differences
Error Definition For Chemistry errors in chemical analysis are simply defined as the difference between a measured value and the true value. in science, measurement error is called experimental error or observational error. It naturally results from the instruments we use, the way we use them, and factors outside our control. errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. error is not an accident or mistake. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. sampling errors arise when a collected sample does not represent the environment being sampled. What is the difference between random and systematic error?
From study.com
How to Calculate Relative Error Chemistry Error Definition For Chemistry in science, measurement error is called experimental error or observational error. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. errors in chemical analysis are simply defined as the difference between a measured value and the true value. What is the difference between random and systematic error? . Error Definition For Chemistry.
From www.youtube.com
Error Definition & Types Chapter 1 Pharmaceutical ChemistryI D Error Definition For Chemistry What is the difference between random and systematic error? It naturally results from the instruments we use, the way we use them, and factors outside our control. errors in chemical analysis are simply defined as the difference between a measured value and the true value. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry. Error Definition For Chemistry.
From www.slideshare.net
Errors in chemical analysis Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. sampling errors arise when a collected sample does not represent the environment being sampled. in science, measurement error is called experimental error or observational error. errors in chemical analysis are simply defined as the difference between a measured. Error Definition For Chemistry.
From www.youtube.com
Analytical Chemistry 1 CH5 L3 Sources of Systematic Error (Slid 4448 Error Definition For Chemistry It naturally results from the instruments we use, the way we use them, and factors outside our control. in science, measurement error is called experimental error or observational error. errors in chemical analysis are simply defined as the difference between a measured value and the true value. sampling errors arise when a collected sample does not represent. Error Definition For Chemistry.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error Definition For Chemistry What is the difference between random and systematic error? Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. . Error Definition For Chemistry.
From www.slideserve.com
PPT Experimental Error PowerPoint Presentation, free download ID Error Definition For Chemistry revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. It naturally results from the instruments we use, the. Error Definition For Chemistry.
From sciencenotes.org
Absolute and Relative Error and How to Calculate Them Error Definition For Chemistry What is the difference between random and systematic error? revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. errors in chemical analysis are simply defined as the difference between a measured value and the true value. It naturally results from the instruments we use,. Error Definition For Chemistry.
From spmphysics.onlinetuition.com.my
Measurement and Error SPM Physics Form 4/Form 5 Revision Notes Error Definition For Chemistry errors in chemical analysis are simply defined as the difference between a measured value and the true value. in science, measurement error is called experimental error or observational error. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. sampling errors arise when. Error Definition For Chemistry.
From www.tessshebaylo.com
Equation For Percent Error In Chemistry Tessshebaylo Error Definition For Chemistry What is the difference between random and systematic error? errors in chemical analysis are simply defined as the difference between a measured value and the true value. sampling errors arise when a collected sample does not represent the environment being sampled. error is not an accident or mistake. revision notes on 1.7.3 error & uncertainty for. Error Definition For Chemistry.
From www.youtube.com
Introduction to Error Analysis for Chemistry Lab YouTube Error Definition For Chemistry in science, measurement error is called experimental error or observational error. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. error is not an accident or mistake. errors in chemical analysis are simply defined as the difference between a measured value and the true value. sampling. Error Definition For Chemistry.
From www.youtube.com
Analytical Chemistry Indeterminate Error Absolute ErrorsMean Errors Error Definition For Chemistry It naturally results from the instruments we use, the way we use them, and factors outside our control. errors in chemical analysis are simply defined as the difference between a measured value and the true value. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my. Error Definition For Chemistry.
From www.youtube.com
Spotting procedural errors in chemistry practicals YouTube Error Definition For Chemistry sampling errors arise when a collected sample does not represent the environment being sampled. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. errors in chemical analysis are simply defined as the difference between a measured value and the true value. revision notes on 1.7.3 error &. Error Definition For Chemistry.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Error Definition For Chemistry What is the difference between random and systematic error? It naturally results from the instruments we use, the way we use them, and factors outside our control. in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. revision notes on 1.7.3 error. Error Definition For Chemistry.
From www.slideserve.com
PPT Analytical Chemistry II PowerPoint Presentation, free download Error Definition For Chemistry It naturally results from the instruments we use, the way we use them, and factors outside our control. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. What is the difference between random and systematic error? revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry. Error Definition For Chemistry.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Error Definition For Chemistry What is the difference between random and systematic error? Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the absolute value of the error, divided by. Error Definition For Chemistry.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Error Definition For Chemistry Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. error is not an accident or mistake. sampling errors arise when a collected sample does not represent the environment being. Error Definition For Chemistry.
From www.sliderbase.com
Significant Figures Presentation Chemistry Error Definition For Chemistry Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. It naturally results from the instruments we use, the way we use them, and factors outside our control. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my. Error Definition For Chemistry.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. What is the difference between random and systematic error? errors in chemical analysis are simply defined as the difference between a measured value and the true value. It naturally results from the instruments we use, the way we use them,. Error Definition For Chemistry.
From eduinput.com
Error in MeasurementDefinition, Types, And Reduction Error Definition For Chemistry in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the absolute value of the error, divided by the. Error Definition For Chemistry.
From www.showme.com
Scientific Notation and Percent Error Science, Chemistry ShowMe Error Definition For Chemistry error is not an accident or mistake. What is the difference between random and systematic error? errors in chemical analysis are simply defined as the difference between a measured value and the true value. It naturally results from the instruments we use, the way we use them, and factors outside our control. the percent error is the. Error Definition For Chemistry.
From www.slideserve.com
PPT Introduction to experimental errors PowerPoint Presentation, free Error Definition For Chemistry Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. in science, measurement error is called experimental error or observational error. sampling errors arise when. Error Definition For Chemistry.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error Definition For Chemistry error is not an accident or mistake. errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. revision. Error Definition For Chemistry.
From www.youtube.com
Percent Error Calculations in Chemistry Regents Chemistry YouTube Error Definition For Chemistry It naturally results from the instruments we use, the way we use them, and factors outside our control. error is not an accident or mistake. What is the difference between random and systematic error? errors in chemical analysis are simply defined as the difference between a measured value and the true value. the percent error is the. Error Definition For Chemistry.
From www.slideserve.com
PPT Experimental Error PowerPoint Presentation, free download ID Error Definition For Chemistry What is the difference between random and systematic error? error is not an accident or mistake. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. revision notes on 1.7.3 error & uncertainty for the edexcel a. Error Definition For Chemistry.
From carreersupport.com
How to Calculate Relative Error with Examples Error Definition For Chemistry Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. sampling errors arise when a collected sample does not represent the environment being sampled. errors in chemical analysis are simply defined as the difference between a measured value and the true value. It naturally results from the instruments we. Error Definition For Chemistry.
From www.youtube.com
Analytical Chemistry 1 CH5 L2 Types of Error in Experimental Data (Slid Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. errors in chemical analysis are simply defined as the difference between a measured value and the true value. sampling errors arise when a collected sample does not represent the environment being sampled. It naturally results from the instruments we. Error Definition For Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. sampling errors arise when a collected sample does not represent the environment being sampled. in science, measurement error is called experimental error or observational error. errors in chemical analysis are simply defined as the difference between a measured. Error Definition For Chemistry.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Error Definition For Chemistry in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Errors that cause the measured mean value (x bar). Error Definition For Chemistry.
From www.showme.com
Percent and Relative Error Science, Chemistry, Physics ShowMe Error Definition For Chemistry in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. What is the difference between random and systematic error? error is not an accident or mistake. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written. Error Definition For Chemistry.
From ksa.mytutorsource.com
Percentage Error Formula, Definition, How to Calculate It! Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. What is the difference between random and systematic error? sampling errors arise when a collected sample does not represent the environment being sampled. errors in chemical analysis are simply defined as the difference between a measured value and the. Error Definition For Chemistry.
From www.expii.com
Types of Error — Overview & Comparison Expii Error Definition For Chemistry What is the difference between random and systematic error? errors in chemical analysis are simply defined as the difference between a measured value and the true value. error is not an accident or mistake. in science, measurement error is called experimental error or observational error. the percent error is the absolute value of the error, divided. Error Definition For Chemistry.
From thechemistrynotes.com
Absolute and Relative Error Definition, Formula, Examples, Differences Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. sampling errors arise when a collected sample does not represent the environment being sampled. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. in. Error Definition For Chemistry.
From sciencenotes.org
Systematic vs Random Error Differences and Examples Error Definition For Chemistry What is the difference between random and systematic error? sampling errors arise when a collected sample does not represent the environment being sampled. the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by. Error Definition For Chemistry.
From dxodaldge.blob.core.windows.net
Definition Of Error In Chemistry at Natasha McKnight blog Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. in science, measurement error is called experimental error or observational error. sampling errors arise when a collected sample does not represent the environment being sampled. error is not an accident or mistake. It naturally results from the instruments. Error Definition For Chemistry.
From dxodaldge.blob.core.windows.net
Definition Of Error In Chemistry at Natasha McKnight blog Error Definition For Chemistry the percent error is the absolute value of the error, divided by the accepted value, and multiplied by. errors in chemical analysis are simply defined as the difference between a measured value and the true value. What is the difference between random and systematic error? It naturally results from the instruments we use, the way we use them,. Error Definition For Chemistry.