Medical Device Fees Canada . the department of health, health canada published the fees in respect of drugs and medical devices order, the. this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application. 11 rows health canada medical device licence application review fees as of april 1, 2014
from medicaldevices.freyrsolutions.com
manufacturers wanting to obtain a medical device licence must complete a medical devices application. Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and medical devices order, the. under the current fees order, health canada charges fees for the examination of medical device. 11 rows health canada medical device licence application review fees as of april 1, 2014
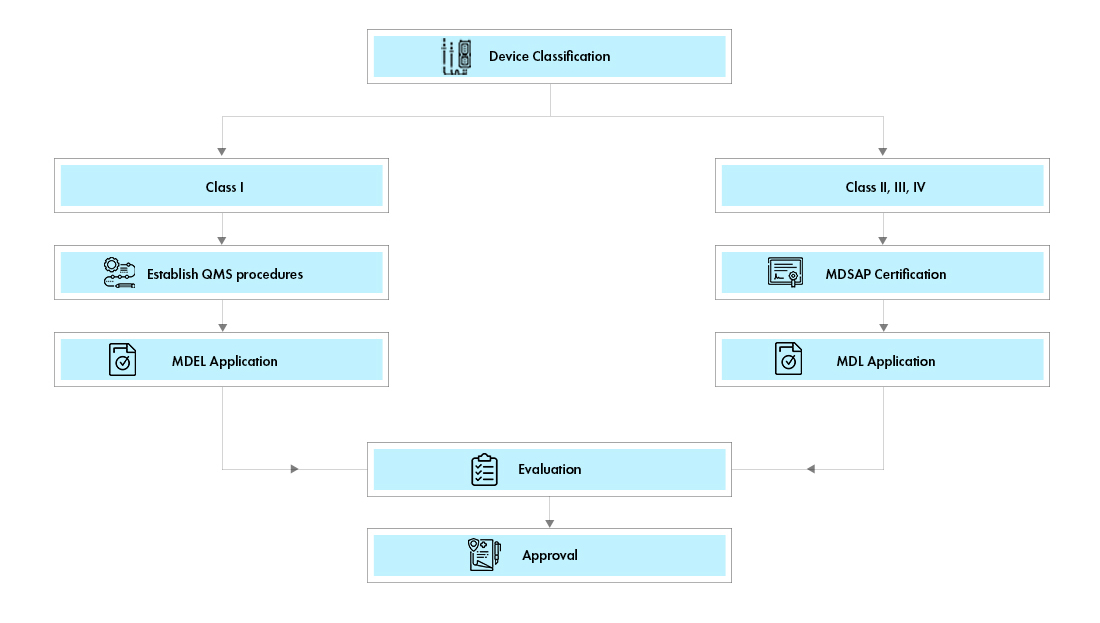
Medical Device Registration Canada, Health Canada, MDSAP certification
Medical Device Fees Canada 11 rows health canada medical device licence application review fees as of april 1, 2014 Fees in respect of drugs and medical devices order. under the current fees order, health canada charges fees for the examination of medical device. this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and medical devices order, the. manufacturers wanting to obtain a medical device licence must complete a medical devices application. 11 rows health canada medical device licence application review fees as of april 1, 2014
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and medical devices order, the. Fees in respect of drugs and medical devices order. 11 rows health canada medical device licence application review fees as. Medical Device Fees Canada.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide Medical Device Fees Canada manufacturers wanting to obtain a medical device licence must complete a medical devices application. under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in.. Medical Device Fees Canada.
From www.canada.ca
Frequently asked questions Medical device establishment licensing and Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and medical devices order, the. 11 rows health canada medical device licence application review fees as of april 1, 2014 manufacturers wanting to obtain. Medical Device Fees Canada.
From www.lookeetech.com
Understanding Health Canada Medical Device Licensing LOOKEETech Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application. 11 rows health canada medical device licence application review fees as of april 1, 2014. Medical Device Fees Canada.
From medicaldeviceacademy.com
Canadian Device Licensing inar Live on May 24, 2017 Medical Device Medical Device Fees Canada the department of health, health canada published the fees in respect of drugs and medical devices order, the. this document provides guidance on how the fee for the right to sell medical devices will be administered in. manufacturers wanting to obtain a medical device licence must complete a medical devices application. 11 rows health canada medical. Medical Device Fees Canada.
From medicaldevices.freyrsolutions.com
Medical Device Registration Canada, Health Canada, MDSAP certification Medical Device Fees Canada manufacturers wanting to obtain a medical device licence must complete a medical devices application. 11 rows health canada medical device licence application review fees as of april 1, 2014 under the current fees order, health canada charges fees for the examination of medical device. this document provides guidance on how the fee for the right to. Medical Device Fees Canada.
From exobbxfun.blob.core.windows.net
Costs Medical Device at Irvin McMorrow blog Medical Device Fees Canada the department of health, health canada published the fees in respect of drugs and medical devices order, the. under the current fees order, health canada charges fees for the examination of medical device. 11 rows health canada medical device licence application review fees as of april 1, 2014 Fees in respect of drugs and medical devices order.. Medical Device Fees Canada.
From bioconcept.com.cn
Canada Medical Device Licence_认证证书_常州百康特医疗器械有限公司 Medical Device Fees Canada the department of health, health canada published the fees in respect of drugs and medical devices order, the. manufacturers wanting to obtain a medical device licence must complete a medical devices application. under the current fees order, health canada charges fees for the examination of medical device. 11 rows health canada medical device licence application review. Medical Device Fees Canada.
From www.qualio.com
How Much Is the FDA Medical Device Registration Fee in 2020? Medical Device Fees Canada under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will be administered in. Fees in respect of drugs and medical devices order.. Medical Device Fees Canada.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Device Fees Canada 11 rows health canada medical device licence application review fees as of april 1, 2014 the department of health, health canada published the fees in respect of drugs and medical devices order, the. under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence. Medical Device Fees Canada.
From www.infoway-inforoute.ca
Infographic The Diffusion of Smart Devices for Health in Canada Medical Device Fees Canada under the current fees order, health canada charges fees for the examination of medical device. 11 rows health canada medical device licence application review fees as of april 1, 2014 Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of. Medical Device Fees Canada.
From medicaldeviceacademy.com
Medical device 510k, quality systems, audits, and training Medical Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application.. Medical Device Fees Canada.
From everycrsreport.com
The FDA Medical Device User Fee Program MDUFA IV Reauthorization Medical Device Fees Canada Fees in respect of drugs and medical devices order. the department of health, health canada published the fees in respect of drugs and medical devices order, the. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will. Medical Device Fees Canada.
From www.regdesk.co
Health Canada Guidance on Private Label Medical Devices RegDesk Medical Device Fees Canada Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in. manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of health, health canada published the fees in respect of drugs and medical. Medical Device Fees Canada.
From www.regdesk.co
Health Canada on Medical Device Shortages RegDesk Medical Device Fees Canada Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of health, health canada published the fees in respect of drugs and medical devices order, the. under the current fees order, health canada charges fees for the examination of medical device. . Medical Device Fees Canada.
From allaboutregulatoryaffairs.com
Manufacturing Import License for New Medical Devices Fee, Validity Medical Device Fees Canada Fees in respect of drugs and medical devices order. 11 rows health canada medical device licence application review fees as of april 1, 2014 this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and. Medical Device Fees Canada.
From www.linkedin.com
New Fees for Medical Devices Medical Device Fees Canada Fees in respect of drugs and medical devices order. under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of health, health canada published the fees in respect of drugs and medical devices order, the. . Medical Device Fees Canada.
From www.canada.ca
Health Canada fees report Fiscal year 2021 to 2022 Canada.ca Medical Device Fees Canada under the current fees order, health canada charges fees for the examination of medical device. the department of health, health canada published the fees in respect of drugs and medical devices order, the. 11 rows health canada medical device licence application review fees as of april 1, 2014 manufacturers wanting to obtain a medical device licence. Medical Device Fees Canada.
From www.lachmanconsultants.com
FDA Announces MDUFA and Outsourcing User Fee Rates for FY 2020 All Medical Device Fees Canada the department of health, health canada published the fees in respect of drugs and medical devices order, the. under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for. Medical Device Fees Canada.
From www.fdahelp.us
Medical Device FDA Registration Fees 2020 Medical Device Fees Canada 11 rows health canada medical device licence application review fees as of april 1, 2014 manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the. Medical Device Fees Canada.
From www.jomakeitfda.com
Medical Device User Fee Rates for Fiscal Year 2023 Medical Device Fees Canada Fees in respect of drugs and medical devices order. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and medical. Medical Device Fees Canada.
From digital.library.unt.edu
The FDA Medical Device User Fee Program MDUFA IV Reauthorization Medical Device Fees Canada under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. the department of health, health canada published the fees in respect of drugs and medical devices order, the. 11 rows health canada medical device licence application review fees as of april 1, 2014. Medical Device Fees Canada.
From www.thema-med.com
CANADA Health Canada increases fees for Medical Devices Thema Med Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. the department of health, health canada published the fees in respect of drugs and. Medical Device Fees Canada.
From sterlingmedicaldevices.com
FDA Medical Device User Fees (MDUFA) for 2024 Medical Device Fees Canada Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the examination of medical device. the department of health, health canada published the fees in respect of drugs and. Medical Device Fees Canada.
From www.regdesk.co
Consultation on Changes to Canadian Medical Device Regulations RegDesk Medical Device Fees Canada under the current fees order, health canada charges fees for the examination of medical device. Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in. 11 rows health canada medical device licence application review fees as of april 1,. Medical Device Fees Canada.
From www.everycrsreport.com
The FDA Medical Device User Fee Program Medical Device Fees Canada 11 rows health canada medical device licence application review fees as of april 1, 2014 under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of health, health canada published the fees in respect of. Medical Device Fees Canada.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Device Fees Canada manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will be administered in. Fees in respect of drugs and medical devices order. 11 rows health canada medical device licence application review fees as of april 1, 2014. Medical Device Fees Canada.
From www.hurondigitalpathology.com
Health Canada Medical Device License TissueScope LE Huron Digital Medical Device Fees Canada Fees in respect of drugs and medical devices order. under the current fees order, health canada charges fees for the examination of medical device. 11 rows health canada medical device licence application review fees as of april 1, 2014 the department of health, health canada published the fees in respect of drugs and medical devices order, the.. Medical Device Fees Canada.
From emmainternational.com
Understanding Medical Device User Fees Medical Device Fees Canada this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the examination of medical device. the department of health, health canada published the fees in respect of drugs and medical devices order, the. manufacturers wanting to obtain. Medical Device Fees Canada.
From www.youtube.com
How to get Medical Device License in Canada Medical Device Sales in Medical Device Fees Canada Fees in respect of drugs and medical devices order. under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides guidance on how the fee for the right to sell medical devices will be administered in.. Medical Device Fees Canada.
From www.qualio.com
How Much Is the FDA Medical Device Registration Fee in 2022? Medical Device Fees Canada Fees in respect of drugs and medical devices order. the department of health, health canada published the fees in respect of drugs and medical devices order, the. 11 rows health canada medical device licence application review fees as of april 1, 2014 this document provides guidance on how the fee for the right to sell medical devices. Medical Device Fees Canada.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Fees Canada the department of health, health canada published the fees in respect of drugs and medical devices order, the. Fees in respect of drugs and medical devices order. this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order, health canada charges fees for the. Medical Device Fees Canada.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Medical Device Fees Canada Fees in respect of drugs and medical devices order. 11 rows health canada medical device licence application review fees as of april 1, 2014 under the current fees order, health canada charges fees for the examination of medical device. manufacturers wanting to obtain a medical device licence must complete a medical devices application. this document provides. Medical Device Fees Canada.
From starfishmedical.com
Canadian medical device industry Medical Device Fees Canada Fees in respect of drugs and medical devices order. under the current fees order, health canada charges fees for the examination of medical device. this document provides guidance on how the fee for the right to sell medical devices will be administered in. the department of health, health canada published the fees in respect of drugs and. Medical Device Fees Canada.
From omcmedical.com
Health Canada Medical Device Listing OMC Medical Medical Device Fees Canada manufacturers wanting to obtain a medical device licence must complete a medical devices application. the department of health, health canada published the fees in respect of drugs and medical devices order, the. this document provides guidance on how the fee for the right to sell medical devices will be administered in. under the current fees order,. Medical Device Fees Canada.