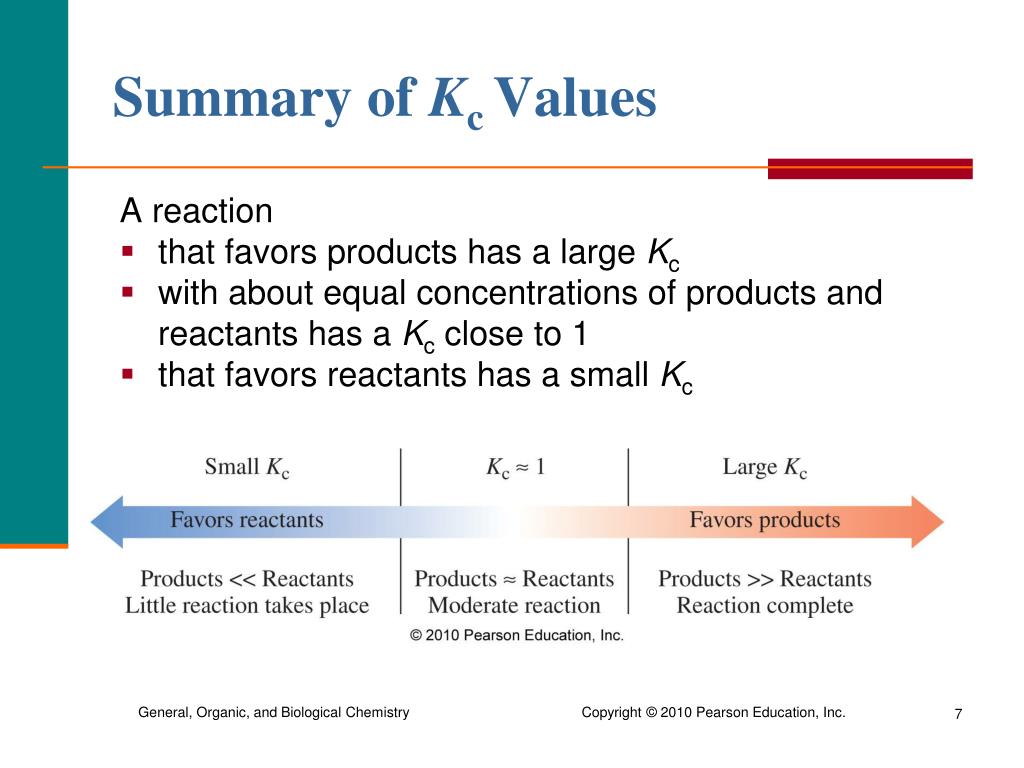
What Does Kc Mean In Equilibrium . In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. K c values are calculated by dividing the concentrations of products by. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. This is the more straightforward case. This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. If \(k\) is a large number, it means. K c in homogeneous equilibria. At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and.
from www.slideserve.com
The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. K c in homogeneous equilibria. K c values are calculated by dividing the concentrations of products by. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. If \(k\) is a large number, it means. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. This is the more straightforward case.
PPT Chapter 9 Chemical Equilibrium PowerPoint Presentation, free
What Does Kc Mean In Equilibrium It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. K c values are calculated by dividing the concentrations of products by. If \(k\) is a large number, it means. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. This is the more straightforward case. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. K c in homogeneous equilibria.
From www.slideserve.com
PPT The Position of Equilibrium PowerPoint Presentation, free What Does Kc Mean In Equilibrium This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: The equilibrium constant of a chemical reaction (usually. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT The Equilibrium Constant PowerPoint Presentation, free download What Does Kc Mean In Equilibrium It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. It applies where everything in the equilibrium mixture. What Does Kc Mean In Equilibrium.
From slidetodoc.com
The Equilibrium Constant Kc Lesson Outline The Equilibrium What Does Kc Mean In Equilibrium K c in homogeneous equilibria. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous. What Does Kc Mean In Equilibrium.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction What Does Kc Mean In Equilibrium This is the more straightforward case. If \(k\) is a large number, it means. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: K c values are calculated by dividing the concentrations of products by. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in. What Does Kc Mean In Equilibrium.
From slideplayer.com
Ch. 13 Fundamental Equilibrium Concepts ppt download What Does Kc Mean In Equilibrium It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K c in homogeneous equilibria. The ratio of the rate constants gives us a new constant,. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Does Kc Mean In Equilibrium If \(k\) is a large number, it means. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. At equilibrium, the forward rate equals the reverse rate (definition of equilibrium):. What Does Kc Mean In Equilibrium.
From www.youtube.com
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice What Does Kc Mean In Equilibrium At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): If \(k\) is a large number, it means. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. The equilibrium constant, k c, is a constant that describes. What Does Kc Mean In Equilibrium.
From www.youtube.com
Equilibrium constant Kc YouTube What Does Kc Mean In Equilibrium It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. K c values are calculated by dividing the concentrations of products by. The magnitude of the equilibrium constant, \(k\), indicates the. What Does Kc Mean In Equilibrium.
From www.chemistrystudent.com
Calculating Equilibrium Constant, Kc (ALevel) ChemistryStudent What Does Kc Mean In Equilibrium If \(k\) is a large number, it means. This is the more straightforward case. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: The equilibrium constant of a chemical reaction (usually denoted. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Chapter 9 Chemical Equilibrium PowerPoint Presentation, free What Does Kc Mean In Equilibrium This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium CHAPTER 15 Chemistry The Molecular Nature What Does Kc Mean In Equilibrium It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. This page defines the equilibrium constant and. What Does Kc Mean In Equilibrium.
From www.showme.com
Qc and Kc in equilibrium changes Science, Chemistry, Physical What Does Kc Mean In Equilibrium The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. In. What Does Kc Mean In Equilibrium.
From www.pinterest.com
K = The Equilibrium Constant. [A] = Molarity of A in the solution. Ap What Does Kc Mean In Equilibrium The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2170548 What Does Kc Mean In Equilibrium This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. If \(k\) is a large number, it means. The equilibrium constant, k c, is a constant that describes the ratio between. What Does Kc Mean In Equilibrium.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium What Does Kc Mean In Equilibrium At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. This is the more straightforward case. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. In order. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID4712265 What Does Kc Mean In Equilibrium K c values are calculated by dividing the concentrations of products by. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. If \(k\) is a large number, it means. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship. What Does Kc Mean In Equilibrium.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps What Does Kc Mean In Equilibrium If \(k\) is a large number, it means. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. It assumes. What Does Kc Mean In Equilibrium.
From www.pinterest.com.mx
Learn how to calculate an equilibrium constant Kc. Chemistry lessons What Does Kc Mean In Equilibrium This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. It applies where everything. What Does Kc Mean In Equilibrium.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 What Does Kc Mean In Equilibrium The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. If \(k\) is a large number, it means. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. In order to quantitatively analyze the equilibrium state of a reaction, we can. What Does Kc Mean In Equilibrium.
From slidetodoc.com
The Equilibrium Constant Kc Lesson Outline The Equilibrium What Does Kc Mean In Equilibrium The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. The ratio of the rate constants gives us a new constant, the. What Does Kc Mean In Equilibrium.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube What Does Kc Mean In Equilibrium This is the more straightforward case. K c in homogeneous equilibria. If \(k\) is a large number, it means. It assumes familiarity with the concept of dynamic equilibrium, as well as the terms homogeneous and heterogeneous as applied to chemical reactions. This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. In. What Does Kc Mean In Equilibrium.
From www.youtube.com
How to Write Equilibrium Constant Expression Kc and Kp Expression What Does Kc Mean In Equilibrium K c in homogeneous equilibria. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. K c values are calculated by dividing the concentrations of products by. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. This page defines the equilibrium constant and introduces the. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Equilibrium Basic Concepts PowerPoint Presentation, free download What Does Kc Mean In Equilibrium This is the more straightforward case. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. This page defines. What Does Kc Mean In Equilibrium.
From www.youtube.com
How to predict the direction of equilibrium by comparing Kc and Qc What Does Kc Mean In Equilibrium It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. If \(k\) is a large number, it means. The. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q What Does Kc Mean In Equilibrium This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. If \(k\) is a large number, it means. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): It assumes familiarity with. What Does Kc Mean In Equilibrium.
From www.youtube.com
What is the equilibrium constant KC Find units of kc Class 10 What Does Kc Mean In Equilibrium The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. If \(k\) is a large number, it means. In order to quantitatively analyze the equilibrium state of a reaction, we can use. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID4712265 What Does Kc Mean In Equilibrium K c in homogeneous equilibria. This is the more straightforward case. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K c values are calculated by dividing the concentrations of products by. In order to quantitatively analyze the equilibrium state of a reaction, we can use the. What Does Kc Mean In Equilibrium.
From chem.libretexts.org
Gas Equilibrium Constants Kc And Kp Chemistry LibreTexts What Does Kc Mean In Equilibrium The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. K c in homogeneous equilibria. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. The magnitude of the equilibrium constant, \(k\), indicates the extent to which a reaction will proceed: K c values are calculated. What Does Kc Mean In Equilibrium.
From www.tes.com
Equilibrium Constant Expression (Kc Kp and units) ALevel Chemistry What Does Kc Mean In Equilibrium K c values are calculated by dividing the concentrations of products by. This page defines the equilibrium constant and introduces the equilibrium constant expressed in terms of concentrations, k c. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. At equilibrium, the forward rate equals the reverse. What Does Kc Mean In Equilibrium.
From materialoster.z21.web.core.windows.net
How To Calculate Kc Equilibrium Constant What Does Kc Mean In Equilibrium If \(k\) is a large number, it means. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The ratio of the rate constants gives us a new constant, the equilibrium constant (k), which. It applies where everything in the equilibrium mixture is present as a gas, or. What Does Kc Mean In Equilibrium.
From slideplayer.com
Introduction to Equilibrium ppt download What Does Kc Mean In Equilibrium In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. K c values are calculated by dividing the concentrations of products by. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in. What Does Kc Mean In Equilibrium.
From slideplayer.com
Introduction to Equilibrium ppt download What Does Kc Mean In Equilibrium K c in homogeneous equilibria. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. This is the more straightforward case. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between. What Does Kc Mean In Equilibrium.
From www.youtube.com
How To Calculate The Equilibrium Constant Kc Practice Problems What Does Kc Mean In Equilibrium At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium.. What Does Kc Mean In Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 What Does Kc Mean In Equilibrium At equilibrium, the forward rate equals the reverse rate (definition of equilibrium): K c in homogeneous equilibria. If \(k\) is a large number, it means. The equilibrium constant, k c, is a constant that describes the ratio between reactants and products at equilibrium. It applies where everything in the equilibrium mixture is present as a gas, or everything is present. What Does Kc Mean In Equilibrium.
From www.slideshare.net
Chapter 15 Lecture Chemical Equilibrium What Does Kc Mean In Equilibrium It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution. In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, keq, or kc, which is a function of the molar concentrations of reactants. This page defines the equilibrium constant and introduces the. What Does Kc Mean In Equilibrium.