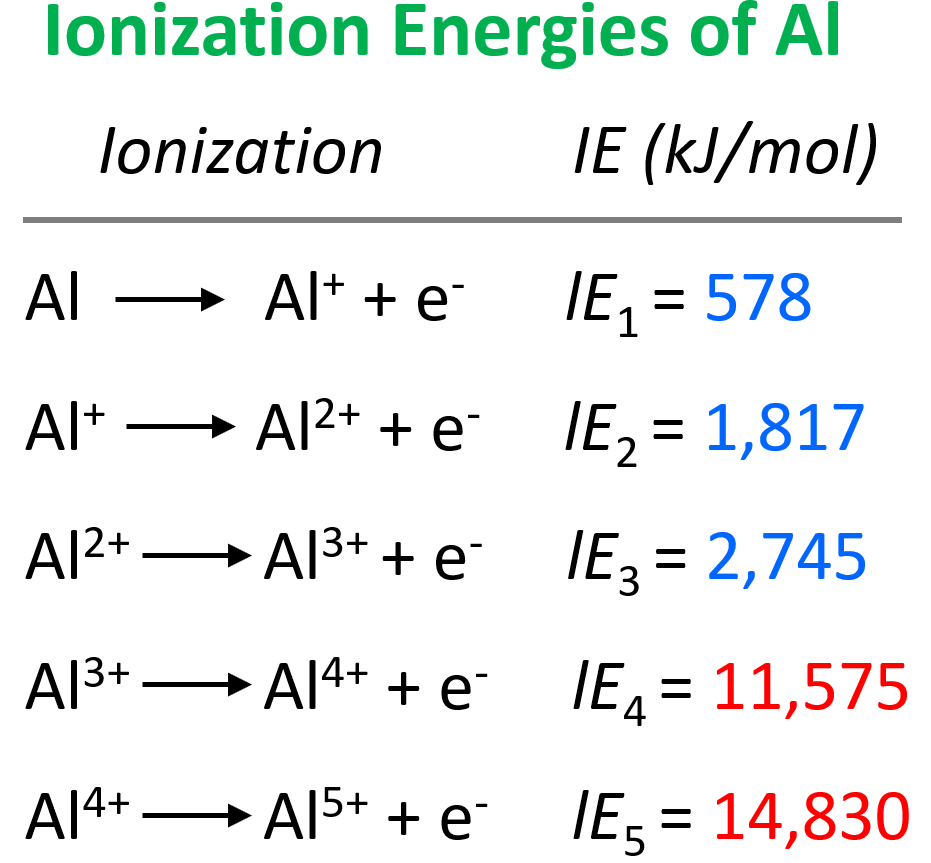
Does Chlorine Have A Higher Ionization Energy Than Sulfur . First ionisation energy generally increases across period 3. This is because first ionisation energies: For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). I i is therefore the energy required for the reaction. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; However, the trend needs a more detailed consideration than the trend in group 2. I suggest that this is very expected as the effective nuclear charge of chloride is higher and. The first ionization energy of chloride is significantly greater than that of sulfur. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Decrease from phosphorus to sulfur then increase again. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Decrease from magnesium to aluminium then increase again, and;
from general.chemistrysteps.com
120 rows ionization energy chart of all the elements is given below. Decrease from phosphorus to sulfur then increase again. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. However, the trend needs a more detailed consideration than the trend in group 2. First ionization energy, second ionization energy as well as. This is because first ionisation energies: The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Periodic trends, arising from the arrangement of the. The first ionization energy of chloride is significantly greater than that of sulfur.
Ionization energy Chemistry Steps
Does Chlorine Have A Higher Ionization Energy Than Sulfur First ionisation energy generally increases across period 3. Decrease from phosphorus to sulfur then increase again. First ionization energy, second ionization energy as well as. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Periodic trends, arising from the arrangement of the. Decrease from magnesium to aluminium then increase again, and; This is because first ionisation energies: For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). First ionisation energy generally increases across period 3. 120 rows ionization energy chart of all the elements is given below. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. I suggest that this is very expected as the effective nuclear charge of chloride is higher and. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; However, the trend needs a more detailed consideration than the trend in group 2. Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. I i is therefore the energy required for the reaction.
From chemistnotes.com
Periodic table Archives Chemistry Notes Does Chlorine Have A Higher Ionization Energy Than Sulfur I i is therefore the energy required for the reaction. The first ionization energy of chloride is significantly greater than that of sulfur. Decrease from phosphorus to sulfur then increase again. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Decrease from magnesium to aluminium then increase again, and; Chemists. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From chem.libretexts.org
7.4 Ionization Energy Chemistry LibreTexts Does Chlorine Have A Higher Ionization Energy Than Sulfur 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; I i is therefore the energy required for the reaction. However, the trend needs a more detailed consideration than the trend in group 2. The. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur First ionisation energy generally increases across period 3. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. This is because first ionisation energies: 120 rows ionization energy chart of. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Does Chlorine Have A Higher Ionization Energy Than Sulfur First ionization energy, second ionization energy as well as. However, the trend needs a more detailed consideration than the trend in group 2. I i is therefore the energy required for the reaction. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). Chemists define the ionization energy (i. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur The first ionization energy of chloride is significantly greater than that of sulfur. Periodic trends, arising from the arrangement of the. Decrease from phosphorus to sulfur then increase again. Decrease from magnesium to aluminium then increase again, and; I i is therefore the energy required for the reaction. However, the trend needs a more detailed consideration than the trend in. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Does Chlorine Have A Higher Ionization Energy Than Sulfur The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; This is because first ionisation energies: First ionisation energy generally increases across period 3. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.numerade.com
SOLVED Sulfur (S) has a slightly lower ionization energy than Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from phosphorus to sulfur then increase again. This is because first ionisation energies: For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Does Chlorine Have A Higher Ionization Energy Than Sulfur I suggest that this is very expected as the effective nuclear charge of chloride is higher and. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Decrease from phosphorus. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.slideserve.com
PPT IB Chemistry ATOMIC THEORY PowerPoint Presentation, free download Does Chlorine Have A Higher Ionization Energy Than Sulfur For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). I i is therefore the energy required for the reaction. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; Chemists define the ionization energy (i i) of an element as the amount of energy. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From askfilo.com
The first ionisation energy of chlorine is greater than that of sulfur.W.. Does Chlorine Have A Higher Ionization Energy Than Sulfur Periodic trends, arising from the arrangement of the. Decrease from magnesium to aluminium then increase again, and; E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. The first ionization energy of chloride is significantly greater than that of sulfur. I suggest that this is very expected as the effective nuclear. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.freepik.com
Premium Vector Chlorine chemical element with first ionization energy Does Chlorine Have A Higher Ionization Energy Than Sulfur Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Decrease from phosphorus to sulfur then increase again. Decrease from magnesium to aluminium then increase again, and; E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. This is because first ionisation energies: I suggest that this is very. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From sofiadonnell.z13.web.core.windows.net
Periodic Table Ionization Energy Chart Does Chlorine Have A Higher Ionization Energy Than Sulfur The first ionization energy of chloride is significantly greater than that of sulfur. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; However, the trend needs a more detailed consideration than the trend in. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Does Chlorine Have A Higher Ionization Energy Than Sulfur This is because first ionisation energies: 120 rows ionization energy chart of all the elements is given below. However, the trend needs a more detailed consideration than the trend in group 2. Decrease from magnesium to aluminium then increase again, and; I i is therefore the energy required for the reaction. For instance, the ionization energy of sodium (alkali metal). Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from phosphorus to sulfur then increase again. 120 rows ionization energy chart of all the elements is given below. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; Periodic trends, arising from the arrangement of the. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From ar.inspiredpencil.com
Ionization Energy List Does Chlorine Have A Higher Ionization Energy Than Sulfur Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. 120 rows ionization energy chart of all the elements is given below. I i is therefore the energy required for the reaction. Decrease from phosphorus to sulfur then increase again.. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from magnesium to aluminium then increase again, and; Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; I suggest that this is very expected as the effective nuclear charge of chloride is higher and. Decrease from phosphorus to sulfur then increase again.. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur I i is therefore the energy required for the reaction. Periodic trends, arising from the arrangement of the. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; First ionisation energy generally increases across period 3. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.toppr.com
Which of the following statements is incorrect? The second ionization Does Chlorine Have A Higher Ionization Energy Than Sulfur Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; 120 rows ionization energy chart of all the elements is given below. Periodic trends, arising from the arrangement of the. This is because first ionisation energies: First ionisation energy generally increases across period 3. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character.. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From slideplayer.com
Trends in Periodic Table ppt download Does Chlorine Have A Higher Ionization Energy Than Sulfur I i is therefore the energy required for the reaction. Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The ionisation energy (ie) of an element is. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.periodic-table.org
Chlorine Ionization Energy Does Chlorine Have A Higher Ionization Energy Than Sulfur 120 rows ionization energy chart of all the elements is given below. Decrease from phosphorus to sulfur then increase again. However, the trend needs a more detailed consideration than the trend in group 2. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Does Chlorine Have A Higher Ionization Energy Than Sulfur The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; Decrease from magnesium to aluminium then increase again, and; However, the trend needs a more detailed consideration than the trend in group 2. Decrease from. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur 120 rows ionization energy chart of all the elements is given below. Decrease from phosphorus to sulfur then increase again. First ionisation energy generally increases across period 3. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.tes.com
Ionisation Energy AS Level Teaching Resources Does Chlorine Have A Higher Ionization Energy Than Sulfur This is because first ionisation energies: The first ionization energy of chloride is significantly greater than that of sulfur. 120 rows ionization energy chart of all the elements is given below. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Does Chlorine Have A Higher Ionization Energy Than Sulfur For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from phosphorus to sulfur then increase again. 120 rows ionization energy chart of all the elements is given below. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. I i is therefore the energy required for the reaction. First ionization energy, second ionization energy as well as. For instance, the ionization energy of sodium (alkali metal). Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Does Chlorine Have A Higher Ionization Energy Than Sulfur This is because first ionisation energies: Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Ionisation energies are measured under standard conditions which are 298 k and. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Does Chlorine Have A Higher Ionization Energy Than Sulfur First ionisation energy generally increases across period 3. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. However, the trend needs a more detailed consideration than the trend in group 2. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). First ionization energy, second ionization. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur This is because first ionisation energies: Chemists define the ionization energy (i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Ionisation energies are measured under standard conditions which are 298 k and 101 kpa; I suggest that this is very expected as the effective. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From mungfali.com
Periodic Table Ionization Energy Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from magnesium to aluminium then increase again, and; First ionisation energy generally increases across period 3. For instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). However, the trend needs a more detailed consideration than the trend in group 2. This is because first ionisation energies: E(g) →. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From commons.wikimedia.org
FileIonization energy atomic size.svg Wikimedia Commons Does Chlorine Have A Higher Ionization Energy Than Sulfur E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. First ionisation energy generally increases across period 3. I suggest that this is very expected as the effective nuclear charge of chloride is higher and. I i is therefore the energy required for the reaction. The ionisation energy (ie) of an. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur This is because first ionisation energies: I i is therefore the energy required for the reaction. The first ionization energy of chloride is significantly greater than that of sulfur. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. Chemists define the ionization energy (i i) of an element as the. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.numerade.com
SOLVED Rank the following elements according to their ionization Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from phosphorus to sulfur then increase again. First ionization energy, second ionization energy as well as. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. I suggest that this is very expected as the effective nuclear charge of chloride is higher and. The ionisation energy (ie) of an element. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From www.chem1.com
Periodic properties of the elements Does Chlorine Have A Higher Ionization Energy Than Sulfur However, the trend needs a more detailed consideration than the trend in group 2. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; Ionisation energies are measured under standard conditions which are 298 k. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Sulfur E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e (g) → e (g) + + e − energy required=i. The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; For instance, the ionization energy of. Does Chlorine Have A Higher Ionization Energy Than Sulfur.
From ar.inspiredpencil.com
Periodic Table Ionization Energy Labeled Does Chlorine Have A Higher Ionization Energy Than Sulfur Decrease from magnesium to aluminium then increase again, and; First ionization energy, second ionization energy as well as. This is because first ionisation energies: The ionisation energy (ie) of an element is the amount of energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ions; I. Does Chlorine Have A Higher Ionization Energy Than Sulfur.