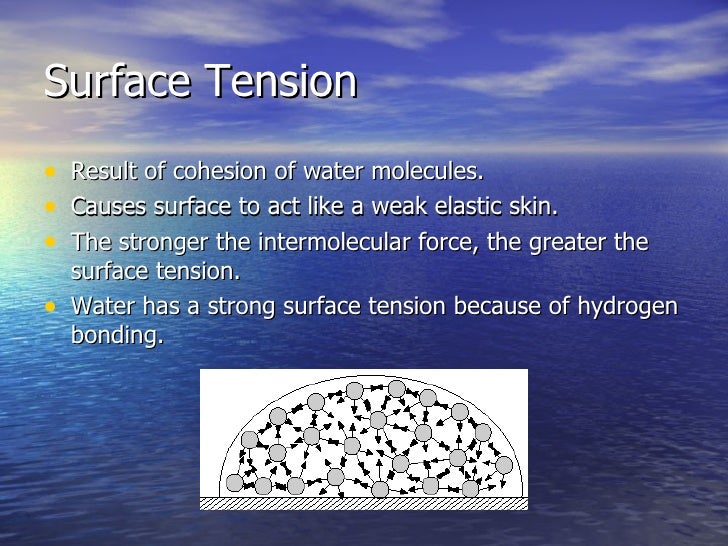
How Do Hydrogen Bonds Cause Surface Tension . The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. At the surface of water, molecules are more. Water molecules are held together by hydrogen bonds, which give water its unique properties. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. The water molecules attract one another due to the water's polar property. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Watch a video of an. Cohesion holds hydrogen bonds together to create surface tension on water.
from www.slideshare.net
Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Cohesion holds hydrogen bonds together to create surface tension on water. Watch a video of an. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Water molecules are held together by hydrogen bonds, which give water its unique properties. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. At the surface of water, molecules are more. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the.
Water, Hydrogen Bonds, and the Hydrologic Cycle
How Do Hydrogen Bonds Cause Surface Tension Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Cohesion holds hydrogen bonds together to create surface tension on water. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Watch a video of an. Water molecules are held together by hydrogen bonds, which give water its unique properties. At the surface of water, molecules are more. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. The water molecules attract one another due to the water's polar property. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain.
From www.slideserve.com
PPT Chemical Properties of Atoms PowerPoint Presentation, free How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. At the surface of water, molecules are more.. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Chapter 3 Biochemistry PowerPoint Presentation, free download How Do Hydrogen Bonds Cause Surface Tension Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Watch a video of an. The water molecules attract one another due to the water's polar property. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. How Do Hydrogen Bonds Cause Surface Tension.
From www.youtube.com
Hydrogen bonding Intermolecular forces and properties AP Chemistry How Do Hydrogen Bonds Cause Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants,. How Do Hydrogen Bonds Cause Surface Tension.
From www.researchgate.net
Schematic diagram of the hydrogen bonding. Download Scientific Diagram How Do Hydrogen Bonds Cause Surface Tension At the surface of water, molecules are more. Watch a video of an. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. The extraordinary stickiness of water is due to. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Water molecules are held together by hydrogen bonds, which give water its unique properties. Since water. How Do Hydrogen Bonds Cause Surface Tension.
From universe-review.ca
Molecules How Do Hydrogen Bonds Cause Surface Tension Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The extraordinary stickiness of water is due to the two hydrogen atoms, which. How Do Hydrogen Bonds Cause Surface Tension.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Do Hydrogen Bonds Cause Surface Tension This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Watch a video of an. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. At the. How Do Hydrogen Bonds Cause Surface Tension.
From mungfali.com
Hydrogen Bonding In Water Diagram How Do Hydrogen Bonds Cause Surface Tension Water molecules are held together by hydrogen bonds, which give water its unique properties. Watch a video of an. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. At the surface of water, molecules are. How Do Hydrogen Bonds Cause Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Do Hydrogen Bonds Cause Surface Tension Cohesion holds hydrogen bonds together to create surface tension on water. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. The. How Do Hydrogen Bonds Cause Surface Tension.
From www.sophia.org
Hydrogen Bonding Definition Tutorial Sophia Learning How Do Hydrogen Bonds Cause Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Water molecules are held together by hydrogen bonds, which give water its unique properties. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and. How Do Hydrogen Bonds Cause Surface Tension.
From slideplayer.com
Chapter 2 Life’s Chemical Basis (Sections ) ppt download How Do Hydrogen Bonds Cause Surface Tension The water molecules attract one another due to the water's polar property. Watch a video of an. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the.. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID How Do Hydrogen Bonds Cause Surface Tension At the surface of water, molecules are more. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The extraordinary stickiness of water is due to the. How Do Hydrogen Bonds Cause Surface Tension.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii How Do Hydrogen Bonds Cause Surface Tension Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Cohesion holds hydrogen bonds together to create surface tension on water. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Watch a. How Do Hydrogen Bonds Cause Surface Tension.
From errantscience.com
Water surface tension ErrantScience How Do Hydrogen Bonds Cause Surface Tension At the surface of water, molecules are more. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. The extraordinary stickiness of water is due to the two hydrogen atoms, which. How Do Hydrogen Bonds Cause Surface Tension.
From www.shutterstock.com
Hydrogen Bonds Surface Tension 库存矢量图(免版税)1640032501 How Do Hydrogen Bonds Cause Surface Tension Water molecules are held together by hydrogen bonds, which give water its unique properties. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. At the surface of water,. How Do Hydrogen Bonds Cause Surface Tension.
From slideplayer.com
Chapter 2 Matter and Energy. ppt download How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. At the surface of water, molecules are more. The water molecules attract one another due to the water's polar property. Water molecules are held together by hydrogen. How Do Hydrogen Bonds Cause Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. How Do Hydrogen Bonds Cause Surface Tension The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Since water is attracted to other molecules, adhesive forces pull the water. How Do Hydrogen Bonds Cause Surface Tension.
From www.chemedx.org
Measuring Surface Tension to Investigate Intermolecular Forces How Do Hydrogen Bonds Cause Surface Tension At the surface of water, molecules are more. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Cohesion holds hydrogen bonds together to create surface tension on water. Surface tension is the energy required to. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Chapter 6 and 7 PowerPoint Presentation, free download ID1957632 How Do Hydrogen Bonds Cause Surface Tension The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. At the surface of water, molecules are more. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. This is why there. How Do Hydrogen Bonds Cause Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Water molecules are held together by hydrogen bonds, which give water its unique properties. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. Surface tension is the energy required. How Do Hydrogen Bonds Cause Surface Tension.
From cehezubl.blob.core.windows.net
Surface Tension Of Liquid Hydrogen at Richard Vice blog How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Cohesion holds hydrogen bonds together to create surface tension on water. Water molecules are held together by hydrogen bonds, which give water its unique properties. Surface tension is the energy required to increase the surface area. How Do Hydrogen Bonds Cause Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube How Do Hydrogen Bonds Cause Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Learn how hydrogen bonds cause water to have high surface tension and how. How Do Hydrogen Bonds Cause Surface Tension.
From www.sliderbase.com
Properties of Water Presentation Biology How Do Hydrogen Bonds Cause Surface Tension Cohesion holds hydrogen bonds together to create surface tension on water. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. The extraordinary stickiness of water is due to the two hydrogen. How Do Hydrogen Bonds Cause Surface Tension.
From chamotgallery.com
Hydrogen Bonding Definition, Types, Effects and Properties (2022) How Do Hydrogen Bonds Cause Surface Tension Cohesion holds hydrogen bonds together to create surface tension on water. Water molecules are held together by hydrogen bonds, which give water its unique properties. Watch a video of an. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. This is why there. How Do Hydrogen Bonds Cause Surface Tension.
From mungfali.com
Hydrogen Bonding Diagram How Do Hydrogen Bonds Cause Surface Tension Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the.. How Do Hydrogen Bonds Cause Surface Tension.
From www.numerade.com
SOLVED 21. What is surface tension? How do hydrogen bonds contribute How Do Hydrogen Bonds Cause Surface Tension The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. The water molecules attract one another due to the water's polar property. At the surface. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID6727958 How Do Hydrogen Bonds Cause Surface Tension At the surface of water, molecules are more. Watch a video of an. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Surface tension is the energy required. How Do Hydrogen Bonds Cause Surface Tension.
From bio1100.nicerweb.com
surface_tension/02_13 How Do Hydrogen Bonds Cause Surface Tension Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Cohesion holds hydrogen bonds together to create surface tension on water. Water molecules are held together by hydrogen bonds, which give water its unique properties. At the surface of water, molecules are more. Learn how hydrogen bonds cause water to have high surface tension and. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideserve.com
PPT Biology Biochemistry Unit Chapter 2 The Chemistry of Life How Do Hydrogen Bonds Cause Surface Tension The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to the. Cohesion holds hydrogen bonds together to create surface tension on water. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The water molecules attract. How Do Hydrogen Bonds Cause Surface Tension.
From www.slideshare.net
Water, Hydrogen Bonds, and the Hydrologic Cycle How Do Hydrogen Bonds Cause Surface Tension Watch a video of an. Cohesion holds hydrogen bonds together to create surface tension on water. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The extraordinary stickiness of water is due to the two hydrogen atoms, which are arranged on one side of the molecule and are attracted to. How Do Hydrogen Bonds Cause Surface Tension.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Do Hydrogen Bonds Cause Surface Tension This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. At the surface of water, molecules are more. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Water molecules are held together by hydrogen bonds, which give water its unique properties.. How Do Hydrogen Bonds Cause Surface Tension.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.5.3 Hydrogen Bonding翰林国际教育 How Do Hydrogen Bonds Cause Surface Tension The water molecules attract one another due to the water's polar property. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to stick together. At the surface of water, molecules are more. Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Watch. How Do Hydrogen Bonds Cause Surface Tension.
From joioycgbl.blob.core.windows.net
Types Of Hydrogen Bonds at Luis Newell blog How Do Hydrogen Bonds Cause Surface Tension Learn how hydrogen bonds cause water to have high surface tension and how this affects capillarity, plants, and rain. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. At the surface of water, molecules are. How Do Hydrogen Bonds Cause Surface Tension.
From delprofedefisica.blogspot.com
Física Tensión superficial How Do Hydrogen Bonds Cause Surface Tension Water molecules are held together by hydrogen bonds, which give water its unique properties. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. This is why there is surface tension and takes a certain amount of energy to break these intermolecular. How Do Hydrogen Bonds Cause Surface Tension.
From sciencenotes.org
Hydrogen Bond Definition and Examples How Do Hydrogen Bonds Cause Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The water molecules attract one another due to the water's polar property. Watch a video of an. Learn how hydrogen bonds cause water to have high surface tension and how this affects. How Do Hydrogen Bonds Cause Surface Tension.