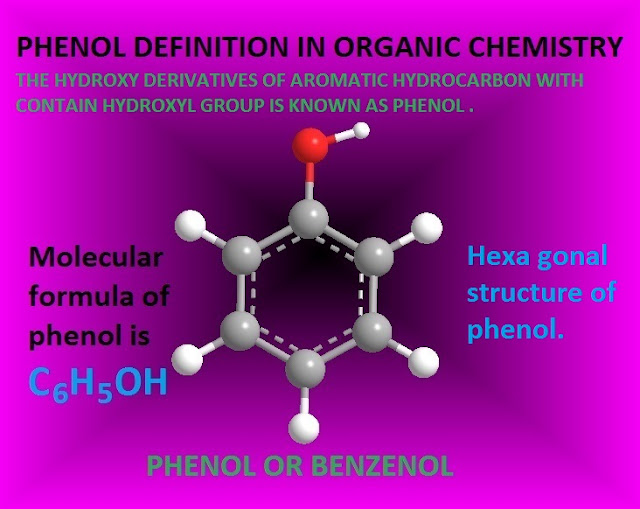
Liquefied Phenol Meaning . Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Explain, in terms of inductive and resonance effects, why a given. What is phenol used for. What is its melting point. The commercial product is a liquid. This page looks at the structure and physical properties of phenol (very old name: Learn its formula, structure, properties, synthesis, and reactions with examples. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. Phenol is the simplest member. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Phenol is both a manufactured chemical and a natural substance. What is phenol or phenolic compound. Phenol has a distinct odor that.
from chemisfast.blogspot.com
Explain why phenols are more acidic than alcohols. What is phenol or phenolic compound. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol is both a manufactured chemical and a natural substance. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Explain the difference in acidity between two given alcohols or phenols. Explain, in terms of inductive and resonance effects, why a given. This page looks at the structure and physical properties of phenol (very old name: Phenol has a distinct odor that. What is phenol used for.
Phenol definitionPhenol structure and Identification in chemistry PG
Liquefied Phenol Meaning The commercial product is a liquid. This page looks at the structure and physical properties of phenol (very old name: Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given. What is its melting point. Phenol is both a manufactured chemical and a natural substance. Explain the difference in acidity between two given alcohols or phenols. Phenol has a distinct odor that. What is phenol or phenolic compound. Learn its formula, structure, properties, synthesis, and reactions with examples. The commercial product is a liquid. What is phenol used for. Phenol is the simplest member. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,.
From www.coursehero.com
[Solved] Calculate the corresponding weights o liquefied phenol and Liquefied Phenol Meaning Phenol is both a manufactured chemical and a natural substance. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Phenol has a distinct odor that. This page looks at the structure and physical properties of phenol (very old name: What is phenol used for. Explain why. Liquefied Phenol Meaning.
From www.biologyonline.com
Phenol coefficient Definition and Examples Biology Online Dictionary Liquefied Phenol Meaning Explain why phenols are more acidic than alcohols. The commercial product is a liquid. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol has a distinct odor that. Phenol is both a manufactured chemical and a natural substance. Explain, in terms of inductive and resonance effects, why a given. What. Liquefied Phenol Meaning.
From ar.inspiredpencil.com
Phenol Liquefied Phenol Meaning This page looks at the structure and physical properties of phenol (very old name: Learn its formula, structure, properties, synthesis, and reactions with examples. What is phenol or phenolic compound. What is its melting point. Explain why phenols are more acidic than alcohols. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the. Liquefied Phenol Meaning.
From ar.inspiredpencil.com
Phenol Liquid Liquefied Phenol Meaning What is phenol or phenolic compound. Explain the difference in acidity between two given alcohols or phenols. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. What is phenol used for. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of. Liquefied Phenol Meaning.
From www.delasco.com
Phenol, Liquefied Delasco Liquefied Phenol Meaning This page looks at the structure and physical properties of phenol (very old name: The commercial product is a liquid. Explain, in terms of inductive and resonance effects, why a given. What is phenol used for. Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol is the simplest member. Phenol is both a manufactured chemical and a natural. Liquefied Phenol Meaning.
From studylib.net
Phenols Liquefied Phenol Meaning Explain why phenols are more acidic than alcohols. Phenol is both a manufactured chemical and a natural substance. What is phenol or phenolic compound. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol is the simplest member. Explain, in terms of inductive and resonance effects, why a given. Learn its. Liquefied Phenol Meaning.
From sidabadi.com
Phenol Liquid PT. Siddharta Sukses Abadi Liquefied Phenol Meaning What is phenol or phenolic compound. Explain why phenols are more acidic than alcohols. This page looks at the structure and physical properties of phenol (very old name: What is its melting point. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Phenol is both a. Liquefied Phenol Meaning.
From chemisfast.blogspot.com
Phenol definitionPhenol structure and Identification in chemistry PG Liquefied Phenol Meaning Phenol is both a manufactured chemical and a natural substance. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. What is its melting point. The commercial product is a liquid. Phenol is. Liquefied Phenol Meaning.
From www.tradeindia.com
Liquid Phenol Application Industrial at Best Price in Mumbai Moksha Liquefied Phenol Meaning Explain, in terms of inductive and resonance effects, why a given. This page looks at the structure and physical properties of phenol (very old name: Explain the difference in acidity between two given alcohols or phenols. Learn its formula, structure, properties, synthesis, and reactions with examples. What is its melting point. What is phenol used for. Explain why phenols are. Liquefied Phenol Meaning.
From www.exportersindia.com
Liquid Phenol at Rs 50 / Kilogram in Delhi ID 6418568 Shri Shakti Liquefied Phenol Meaning Explain the difference in acidity between two given alcohols or phenols. Explain, in terms of inductive and resonance effects, why a given. What is phenol used for. What is phenol or phenolic compound. Explain why phenols are more acidic than alcohols. What is its melting point. This page looks at the structure and physical properties of phenol (very old name:. Liquefied Phenol Meaning.
From www.lambsmead.com
LIQUIFIED PHENOL AQUEOUS SOLUTION 80 WW LAMBSMEAD LIMITED Liquefied Phenol Meaning Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Phenol is the simplest member. Explain the difference in acidity between two given alcohols or phenols. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. This page looks. Liquefied Phenol Meaning.
From www.restaurarconservar.com
HPLC Phenol Liquified and Pure Your online store Liquefied Phenol Meaning Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol is both a manufactured chemical and a natural substance. Phenol is the simplest member. Explain the difference in acidity between two given alcohols or phenols. The commercial product is a liquid. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon. Liquefied Phenol Meaning.
From www.tradeindia.com
Liquid Phenol Molecular Formula C6h6o at Best Price in Ankleshwar Liquefied Phenol Meaning Explain the difference in acidity between two given alcohols or phenols. The commercial product is a liquid. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. What is phenol used for. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of. Liquefied Phenol Meaning.
From www.medicalsearch.com.au
Liquid Phenol 80 Percent W/W 100ml AA080PHELIQ1 Liquefied Phenol Meaning What is phenol or phenolic compound. Phenol has a distinct odor that. Phenol is both a manufactured chemical and a natural substance. This page looks at the structure and physical properties of phenol (very old name: Explain the difference in acidity between two given alcohols or phenols. Phenol is the simplest member. The commercial product is a liquid. Learn its. Liquefied Phenol Meaning.
From capitalresin.com
The Physical and Chemical Properties of Phenol Liquefied Phenol Meaning Explain the difference in acidity between two given alcohols or phenols. Phenol is both a manufactured chemical and a natural substance. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given. What is phenol or phenolic compound. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly. Liquefied Phenol Meaning.
From sielc.com
Phenol SIELC Technologies Liquefied Phenol Meaning Phenol has a distinct odor that. What is phenol used for. Explain why phenols are more acidic than alcohols. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. The commercial product is a liquid. Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol is the simplest member. Phenol are. Liquefied Phenol Meaning.
From www.sssaustralia.com.au
Phenol Liquid 80 BP 100ml SSS Australia SSS Australia Medical Liquefied Phenol Meaning What is phenol or phenolic compound. Phenol is both a manufactured chemical and a natural substance. Explain, in terms of inductive and resonance effects, why a given. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. This page looks at the structure and physical properties of. Liquefied Phenol Meaning.
From www.youtube.com
Phenol Resonance and Acidity YouTube Liquefied Phenol Meaning What is phenol or phenolic compound. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol is both a manufactured chemical and a natural substance. The commercial product is a liquid. Explain why phenols are more acidic than alcohols. Phenol has a distinct odor that. Explain the difference in acidity between. Liquefied Phenol Meaning.
From www.indiamart.com
Red Liquid Phenol at Rs 155/litre Phenol Liquid in Alwaye ID Liquefied Phenol Meaning Explain why phenols are more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Explain, in terms of inductive and resonance effects, why a given. Phenol has a distinct odor that. Learn. Liquefied Phenol Meaning.
From www.fishersci.com
Phenol, Liquefied, 89, Spectrum Chemical, Quantity 120 mL Fisher Liquefied Phenol Meaning What is its melting point. What is phenol used for. Explain, in terms of inductive and resonance effects, why a given. Learn its formula, structure, properties, synthesis, and reactions with examples. What is phenol or phenolic compound. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol is the simplest member.. Liquefied Phenol Meaning.
From www.slideserve.com
PPT Phenols PowerPoint Presentation, free download ID735552 Liquefied Phenol Meaning Explain, in terms of inductive and resonance effects, why a given. Explain the difference in acidity between two given alcohols or phenols. What is phenol used for. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached. Liquefied Phenol Meaning.
From pediaa.com
Difference Between Phenol and Phenyl Definition, Structure, Properties Liquefied Phenol Meaning Phenol is the simplest member. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol is both a manufactured chemical and a natural substance. Phenol has a distinct odor that. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the. Liquefied Phenol Meaning.
From chemistnotes.com
Phenol Definition, Classification, properties, Preparation, and 7 Liquefied Phenol Meaning Explain, in terms of inductive and resonance effects, why a given. Phenol is the simplest member. Phenol is both a manufactured chemical and a natural substance. Explain why phenols are more acidic than alcohols. The commercial product is a liquid. Explain the difference in acidity between two given alcohols or phenols. What is its melting point. Learn its formula, structure,. Liquefied Phenol Meaning.
From chemisfast.blogspot.com
Phenol definitionPhenol structure and Identification in chemistry PG Liquefied Phenol Meaning Phenol is both a manufactured chemical and a natural substance. Phenol has a distinct odor that. Phenol is the simplest member. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. What is phenol used for. What is its melting point. Explain the difference in acidity between. Liquefied Phenol Meaning.
From www.fishersci.ca
Phenol, Liquefied, 89, Spectrum™ Chemical Fisher Scientific Liquefied Phenol Meaning The commercial product is a liquid. Explain the difference in acidity between two given alcohols or phenols. Phenol is the simplest member. What is its melting point. Phenol has a distinct odor that. Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the. Liquefied Phenol Meaning.
From chemisfast.blogspot.com
Phenol definitionPhenol structure and Identification in chemistry PG Liquefied Phenol Meaning Explain the difference in acidity between two given alcohols or phenols. Phenol has a distinct odor that. What is phenol used for. The commercial product is a liquid. Phenol is the simplest member. Phenol is both a manufactured chemical and a natural substance. This page looks at the structure and physical properties of phenol (very old name: Explain, in terms. Liquefied Phenol Meaning.
From www.pccarx.com
PHENOL LIQUEFIED USP PCCA Liquefied Phenol Meaning Phenol has a distinct odor that. What is its melting point. This page looks at the structure and physical properties of phenol (very old name: Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Explain, in terms of inductive and resonance effects, why a given. The. Liquefied Phenol Meaning.
From www.carolina.com
Phenol Liquefied, Laboratory Grade, 500 mL Liquefied Phenol Meaning Phenol is the simplest member. Explain why phenols are more acidic than alcohols. Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol is both a manufactured chemical and a natural substance. The commercial product is a liquid. What is phenol used for. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to. Liquefied Phenol Meaning.
From ar.inspiredpencil.com
Phenol Liquid Liquefied Phenol Meaning Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. What is its melting point. What is phenol or phenolic compound. The commercial product is a liquid. Phenol has a distinct odor that. Explain why phenols are. Liquefied Phenol Meaning.
From www.indiamart.com
Pure Liquid Phenol at Rs 75/kg Liquid Phenol in Bhubaneswar ID Liquefied Phenol Meaning Learn its formula, structure, properties, synthesis, and reactions with examples. Phenol is the simplest member. What is its melting point. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Phenol is both a manufactured chemical and a natural substance. Phenol has a distinct odor that. Explain,. Liquefied Phenol Meaning.
From www.chem.ucalgary.ca
Chapter 24 Phenols Liquefied Phenol Meaning What is its melting point. Phenol is both a manufactured chemical and a natural substance. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. This page looks at the structure and physical properties of phenol (very old name: Learn its formula, structure, properties, synthesis, and reactions with examples. What is phenol. Liquefied Phenol Meaning.
From mungfali.com
Phenol Lewis Structure Liquefied Phenol Meaning What is its melting point. Phenol is both a manufactured chemical and a natural substance. Phenol has a distinct odor that. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. Explain, in terms of inductive and resonance effects, why a given. Explain the difference in acidity. Liquefied Phenol Meaning.
From www.spbotanicals.com
Liquefied Phenol Solution 10 Carbolic Acid Lab Grade — Sun Pure Liquefied Phenol Meaning This page looks at the structure and physical properties of phenol (very old name: Explain the difference in acidity between two given alcohols or phenols. What is phenol or phenolic compound. What is its melting point. Phenol has a distinct odor that. Learn its formula, structure, properties, synthesis, and reactions with examples. What is phenol used for. Phenol is both. Liquefied Phenol Meaning.
From chemistnotes.com
Phenol Definition, Classification, properties, Preparation, and 7 Liquefied Phenol Meaning Explain why phenols are more acidic than alcohols. Learn its formula, structure, properties, synthesis, and reactions with examples. What is phenol or phenolic compound. Phenol is both a manufactured chemical and a natural substance. Phenol liquid is often used in molecular biology with trichloromethane and chloroform to separate rna, dna, or proteins,. Phenol are hydroxy derivatives of aromatic hydrocarbons in. Liquefied Phenol Meaning.
From www.aquaportail.com
Phénol définition et explications Liquefied Phenol Meaning Explain, in terms of inductive and resonance effects, why a given. What is phenol used for. This page looks at the structure and physical properties of phenol (very old name: What is its melting point. Phenol is the simplest member. Phenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of. Liquefied Phenol Meaning.