Acid Base Titration Lab Answers . titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. The analyte (titrand) is the solution with an unknown molarity. Study with quizlet and memorize. to use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the.
from www.chegg.com
titration is an analytical quantitative technique used to determine the concentration of a solute; to use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration. Study with quizlet and memorize. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. The analyte (titrand) is the solution with an unknown molarity.
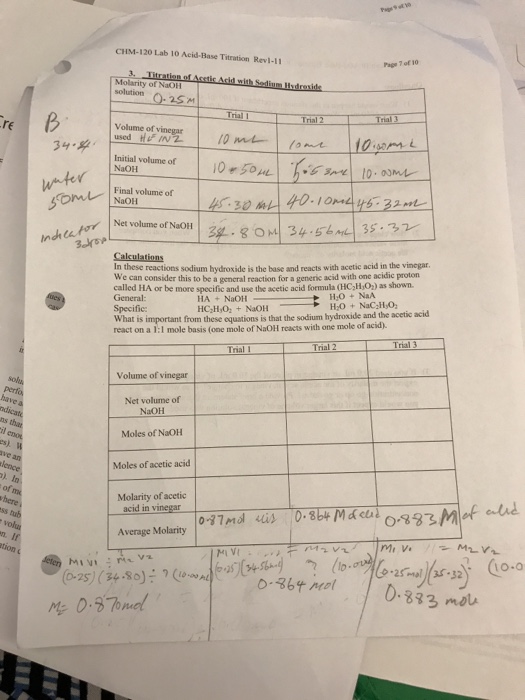
Solved CHIM120 Lab 10 AcidBase Titration Revi11 Molarity
Acid Base Titration Lab Answers Study with quizlet and memorize. Study with quizlet and memorize. to use a previously standardized solution to determine the concentration of an unknown acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is an analytical quantitative technique used to determine the concentration of a solute; The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. Be able to determine the k a or k b from ph data associated with the titration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers to use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an. Acid Base Titration Lab Answers.
From www.docsity.com
Acid base titration lab answers Docsity Acid Base Titration Lab Answers a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is an analytical quantitative technique used to determine the concentration of a solute; to use a previously standardized solution to determine the concentration of an unknown acid. The purpose of this experiment is to observe. Acid Base Titration Lab Answers.
From 2021notubenju.blogspot.com
[11+] Acid Base Review Answers Important Questions Of Acid And Bases Acid Base Titration Lab Answers a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. to use a previously standardized solution to determine the concentration of an unknown acid.. Acid Base Titration Lab Answers.
From www.chegg.com
Solved PART B Acid Base Titration Lab Report I Acid Base Titration Lab Answers The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. Be able to determine the k a or k b from ph data associated with the titration. Study with quizlet and memorize. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Acid Base Titration Lab Answers.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Acid Base Titration Lab Answers The reagent (titrant) is the solution with a known molarity that will react with the analyte. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. titration is an analytical quantitative technique used to determine the concentration of a solute; to use a previously standardized solution to determine the concentration. Acid Base Titration Lab Answers.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Acid Base Titration Lab Answers The analyte (titrand) is the solution with an unknown molarity. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. to use a previously. Acid Base Titration Lab Answers.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Lab Answers The reagent (titrant) is the solution with a known molarity that will react with the analyte. to use a previously standardized solution to determine the concentration of an unknown acid. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. Study with quizlet and. Acid Base Titration Lab Answers.
From avery-yersbloglester.blogspot.com
Acid Base Titration Lab Report Answers Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. to use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated. Acid Base Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration. Study with quizlet and memorize. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine the accurate concentration of a. Acid Base Titration Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it. Acid Base Titration Lab Answers.
From vasastephaniemackay.blogspot.com
Acid Base Titration Lab Report Answers Stephanie Mackay Acid Base Titration Lab Answers a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. to use a previously standardized solution to determine the concentration of an unknown acid.. Acid Base Titration Lab Answers.
From www.transtutors.com
(Get Answer) LAB REPORT SHEET AcidBase Titration 20 50 A. Concentration Of... Transtutors Acid Base Titration Lab Answers The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the. Acid Base Titration Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 10 A. Acid Base Titration Lab Answers in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the solution with an unknown molarity. Study with quizlet and memorize.. Acid Base Titration Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize. Be able to determine the k a or k b from ph data associated with the titration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure. Acid Base Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the k a or k b from ph data associated with the titration. a. Acid Base Titration Lab Answers.
From www.vecteezy.com
Acid base titration experiment and phases of color change during titration 20240684 Vector Art Acid Base Titration Lab Answers to use a previously standardized solution to determine the concentration of an unknown acid. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the k a or k b from ph data associated with the titration. in this lab, we will be learning about titration curves, equivalence. Acid Base Titration Lab Answers.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Acid Base Titration Lab Answers a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Study with quizlet and memorize. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. titration is an analytical quantitative technique used to determine. Acid Base Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 20 1. Brand Acid Base Titration Lab Answers to use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize. The analyte (titrand) is the solution with an unknown molarity. titration is an analytical quantitative technique used to determine the concentration of a solute; in this lab, we will be learning about titration curves, equivalence points and endpoints. Acid Base Titration Lab Answers.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Acid Base Titration Lab Answers in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Study with quizlet and memorize. The analyte (titrand) is the solution with an unknown molarity.. Acid Base Titration Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize. The reagent (titrant) is the solution with a known molarity that will react with the analyte. . Acid Base Titration Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. Study with quizlet and memorize. The reagent (titrant) is the solution with a known molarity that will. Acid Base Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Answers to use a previously standardized solution to determine the concentration of an unknown acid. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize. The reagent. Acid Base Titration Lab Answers.
From www.chegg.com
Solved CHIM120 Lab 10 AcidBase Titration Revi11 Molarity Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be. Acid Base Titration Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Explanation Doc [1.6mb Acid Base Titration Lab Answers The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. to use a previously standardized solution to determine the concentration of an unknown acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it. Acid Base Titration Lab Answers.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Acid Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration. Study with quizlet and memorize. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. The analyte (titrand) is the solution with an unknown molarity. The purpose of this experiment. Acid Base Titration Lab Answers.
From www.vrogue.co
Acid Base Titration Lab Questions Answers vrogue.co Acid Base Titration Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the k. Acid Base Titration Lab Answers.
From studyfinder.org
Unveiling Acid Base Titration Pre Lab Answers A Comprehensive Guide Acid Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration. titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate. Acid Base Titration Lab Answers.
From www.chegg.com
Solved REPORT EXPERIMENT 9 ACID BASE TITRATION REPORT Acid Base Titration Lab Answers The reagent (titrant) is the solution with a known molarity that will react with the analyte. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. The analyte (titrand) is the solution with an unknown molarity. in this lab, we will be learning about titration curves, equivalence points and endpoints as. Acid Base Titration Lab Answers.
From kyrakruwesparza.blogspot.com
Acidbase Titration Lab Report Answers Hcl and Naoh KyrakruwEsparza Acid Base Titration Lab Answers The analyte (titrand) is the solution with an unknown molarity. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. The reagent (titrant) is the solution with a known. Acid Base Titration Lab Answers.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Acid Base Titration Lab Answers a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the. Acid Base Titration Lab Answers.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Acid Base Titration Lab Answers The analyte (titrand) is the solution with an unknown molarity. Be able to determine the k a or k b from ph data associated with the titration. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between the. a titration is an analytical procedure used to determine. Acid Base Titration Lab Answers.
From exoxjybnj.blob.core.windows.net
Acid Base Titration Lab Introduction at Nichole Brumback blog Acid Base Titration Lab Answers The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in this lab, we will be learning about titration curves, equivalence points and endpoints as well as understanding the relationship between. Acid Base Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. to use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize. titration is an analytical. Acid Base Titration Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers Study with quizlet and memorize. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. to use a previously standardized solution to determine the concentration of an unknown acid. in. Acid Base Titration Lab Answers.
From www.vrogue.co
Acid Base Titration Lab Answers vrogue.co Acid Base Titration Lab Answers Study with quizlet and memorize. Be able to determine the k a or k b from ph data associated with the titration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.. Acid Base Titration Lab Answers.