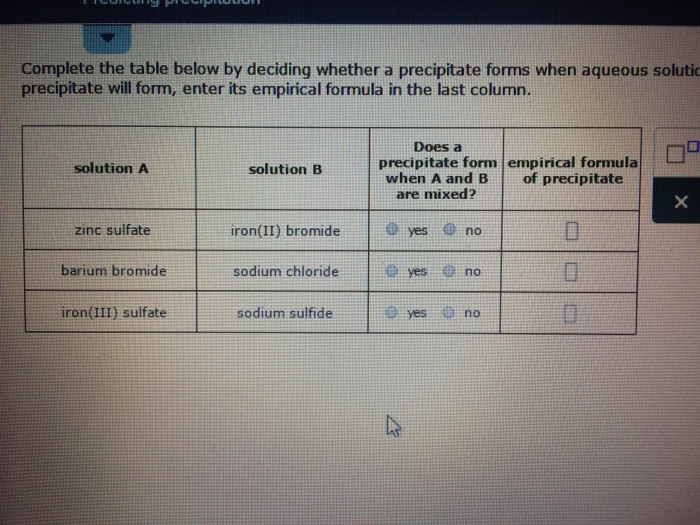
Zinc Chloride And Potassium Sulfide Precipitate . We described a precipitation reaction in which a colorless solution of. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams of zinc(ii) nitrate and sodium sulfide. Enter an equation of an ionic chemical equation and press the balance button. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Sodium chloride, sodium hydroxide, or sodium sulfate?. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: This guide will show how to use the solubility rules. You can use ammonia solution instead of sodium hydroxide. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate.
from www.chegg.com
How many grams of zinc(ii) nitrate and sodium sulfide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. You can use ammonia solution instead of sodium hydroxide. Enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide, or sodium sulfate?. We described a precipitation reaction in which a colorless solution of. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample:
Solved Complete the table below by deciding whether a
Zinc Chloride And Potassium Sulfide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. How many grams of zinc(ii) nitrate and sodium sulfide. You can use ammonia solution instead of sodium hydroxide. Enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide, or sodium sulfate?. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: We described a precipitation reaction in which a colorless solution of. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate.
From www.slideshare.net
Precipitates Zinc Chloride And Potassium Sulfide Precipitate You can use ammonia solution instead of sodium hydroxide. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. How many grams of zinc(ii) nitrate and sodium sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Adding 10.0 ml of a dilute solution of zinc nitrate. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Chloride And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. How many grams of zinc(ii) nitrate and sodium sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. You can use ammonia solution instead of sodium hydroxide. When two aqueous solutions of ionic. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Zinc Chloride And Potassium Sulfide Precipitate What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use. Zinc Chloride And Potassium Sulfide Precipitate.
From questions.kunduz.com
Complete the table below by deciding whet... Physical Chemistry Zinc Chloride And Potassium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: How many grams of zinc(ii) nitrate and sodium sulfide. This guide will show how to use the solubility rules. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. You can use ammonia solution instead. Zinc Chloride And Potassium Sulfide Precipitate.
From www.youtube.com
Double displacement of ZnSO4 + BaCl2 Zinc sulphate + Barium chloride Zinc Chloride And Potassium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Enter an equation of an ionic chemical equation and press the balance button. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Zinc Chloride And Potassium Sulfide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Chloride And Potassium Sulfide Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. We described a precipitation reaction in which a colorless solution of. How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when. Zinc Chloride And Potassium Sulfide Precipitate.
From www.shutterstock.com
79 Residual Volume Images, Stock Photos & Vectors Shutterstock Zinc Chloride And Potassium Sulfide Precipitate We described a precipitation reaction in which a colorless solution of. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Which solution could be used to precipitate the. Zinc Chloride And Potassium Sulfide Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Zinc Chloride And Potassium Sulfide Precipitate We described a precipitation reaction in which a colorless solution of. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Sodium chloride, sodium hydroxide, or sodium sulfate?. This guide will show how to use. Zinc Chloride And Potassium Sulfide Precipitate.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams. Zinc Chloride And Potassium Sulfide Precipitate.
From hydrogenpotanezu.blogspot.com
Hydrogen Zinc Chloride Plus Hydrogen Sulfide Zinc Chloride And Potassium Sulfide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready. Zinc Chloride And Potassium Sulfide Precipitate.
From www.slideshare.net
Precipitation react2 Zinc Chloride And Potassium Sulfide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. This guide will show how to use the solubility rules. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: A typical precipitation reaction occurs. Zinc Chloride And Potassium Sulfide Precipitate.
From www.toppr.com
Given below are word equations. Convert them into chemical equations Zinc Chloride And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. How many grams of zinc(ii) nitrate. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether a precipitate Zinc Chloride And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. We described a precipitation reaction in which a colorless solution of. This guide will show how to use the solubility rules. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Adding 10.0 ml of. Zinc Chloride And Potassium Sulfide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Chloride And Potassium Sulfide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. We described a precipitation reaction in which a colorless solution of. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Adding 10.0 ml of a. Zinc Chloride And Potassium Sulfide Precipitate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Chloride And Potassium Sulfide Precipitate This guide will show how to use the solubility rules. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml of. Zinc Chloride And Potassium Sulfide Precipitate.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Does Silver Nitrate and Potassium Sulfide precipitate when Zinc Chloride And Potassium Sulfide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: This guide will show how to use the solubility rules. What. Zinc Chloride And Potassium Sulfide Precipitate.
From brainly.in
Just write the chemical formula of these compounds/radicals1 Zinc Chloride And Potassium Sulfide Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or sodium sulfate?. You can use ammonia solution instead of sodium hydroxide. A typical precipitation reaction occurs when an aqueous solution of barium chloride. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED When aqueous solutions of potassium phosphate and zinc(II Zinc Chloride And Potassium Sulfide Precipitate We described a precipitation reaction in which a colorless solution of. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: This guide will show how to use the solubility rules. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. How many grams of zinc(ii) nitrate. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Chloride And Potassium Sulfide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when an aqueous solution. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Zinc Chloride And Potassium Sulfide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. This guide will show how to use the solubility rules. When two aqueous solutions of ionic compounds. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below bv deciding whether precipitate forms Zinc Chloride And Potassium Sulfide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. How many grams of zinc(ii) nitrate and sodium sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Predicting precipitation Complete the table below by Zinc Chloride And Potassium Sulfide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. We described a precipitation reaction in which a colorless solution of. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Enter an equation of an ionic chemical equation and press the balance button. Which solution could be used to precipitate the barium ion,. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Chloride And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. How many grams of zinc(ii) nitrate and sodium sulfide. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of. Adding 10.0 ml of a dilute solution of zinc nitrate to. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Chloride And Potassium Sulfide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. This guide will show how to use the solubility rules. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. How many grams of. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Zinc Chloride And Potassium Sulfide Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. We described a precipitation reaction in which a colorless solution of. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Zinc Chloride And Potassium Sulfide Precipitate.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. You can use ammonia solution instead of sodium hydroxide. Which solution. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVEDIf aqueous solutions of potassium sulfide and iron(III) chloride Zinc Chloride And Potassium Sulfide Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. You can use ammonia solution instead of sodium hydroxide. Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. We described a precipitation reaction in which a colorless solution of. How. Zinc Chloride And Potassium Sulfide Precipitate.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Precipitate We described a precipitation reaction in which a colorless solution of. You can use ammonia solution instead of sodium hydroxide. How many grams of zinc(ii) nitrate and sodium sulfide. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium. Zinc Chloride And Potassium Sulfide Precipitate.
From questions.kunduz.com
Complete the table below by deciding whe... Chemistry Zinc Chloride And Potassium Sulfide Precipitate This guide will show how to use the solubility rules. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. You can use ammonia solution instead of sodium hydroxide. We described a precipitation reaction in which a colorless solution of. Enter an equation of an ionic chemical equation and press the. Zinc Chloride And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Chloride And Potassium Sulfide Precipitate We described a precipitation reaction in which a colorless solution of. This guide will show how to use the solubility rules. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Chloride And Potassium Sulfide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium chloride, sodium hydroxide, or sodium sulfate?. You can use ammonia solution instead of sodium hydroxide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Predicting precipitation Complete the table below by Zinc Chloride And Potassium Sulfide Precipitate 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. You can use ammonia solution instead of sodium hydroxide. Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml. Zinc Chloride And Potassium Sulfide Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Zinc Chloride And Potassium Sulfide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. This guide will show how to use the solubility rules. We described a precipitation reaction in which a colorless solution of. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: You can use ammonia solution instead of. Zinc Chloride And Potassium Sulfide Precipitate.
From www.slideshare.net
Precipitates Zinc Chloride And Potassium Sulfide Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Enter an equation of an ionic. Zinc Chloride And Potassium Sulfide Precipitate.