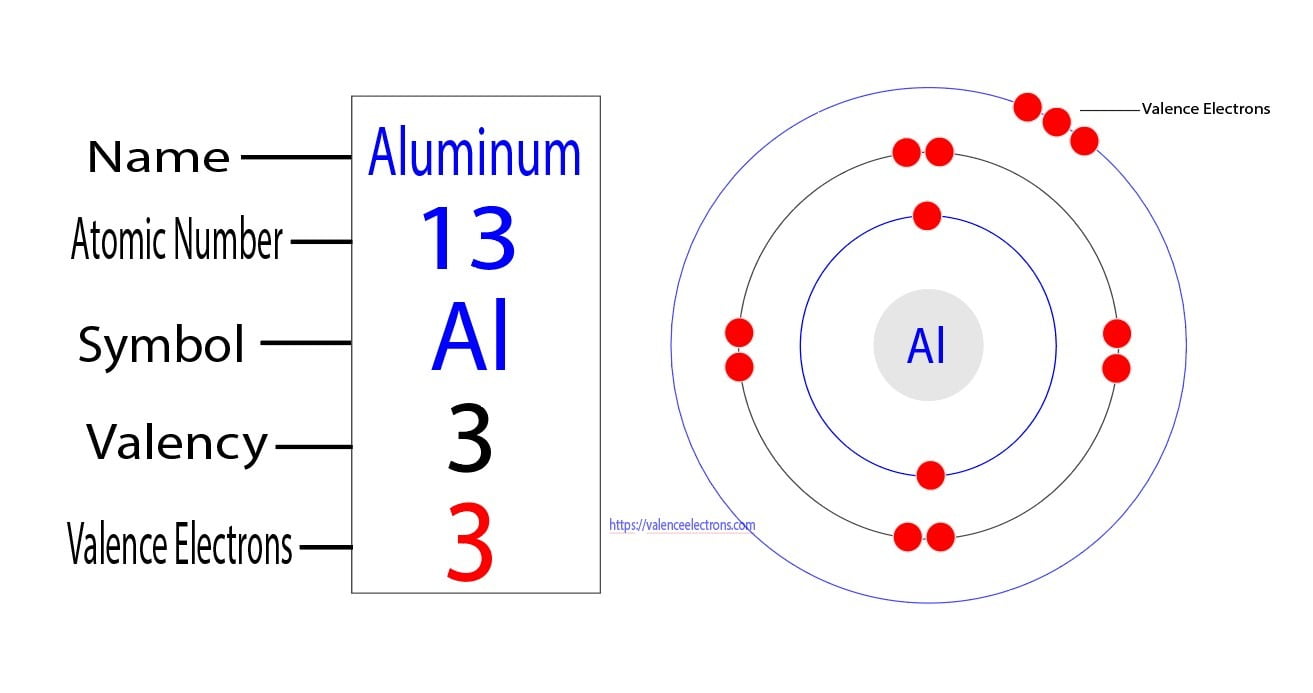
Aluminum Atom Protons Neutrons Electrons . This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Why is aluminum in period 3? Describe the locations, charges, and masses of the three main subatomic particles. It is the only primordial aluminium isotope, i.e. How many protons, neutrons, and electrons does aluminum contain? Therefore, an aluminum atom has fourteen neutrons. Hence, it lies in group 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of 26.98154 and a mass. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Determine the number of protons and. Aluminum (al) has an atomic number of 13, which means it has 13. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The difference between the mass number of the aluminum atom and the number of protons is fourteen.
from elchoroukhost.net
It has an atomic weight of 26.98154 and a mass. Why is aluminum in period 3? How many protons, neutrons, and electrons does aluminum contain? Hence, it lies in group 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Therefore, an aluminum atom has fourteen neutrons. Describe the locations, charges, and masses of the three main subatomic particles. The difference between the mass number of the aluminum atom and the number of protons is fourteen. It is the only primordial aluminium isotope, i.e.
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table
Aluminum Atom Protons Neutrons Electrons Hence, it lies in group 13. It has an atomic weight of 26.98154 and a mass. The difference between the mass number of the aluminum atom and the number of protons is fourteen. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. How many protons, neutrons, and electrons does aluminum contain? It is the only primordial aluminium isotope, i.e. Describe the locations, charges, and masses of the three main subatomic particles. Aluminum (al) has an atomic number of 13, which means it has 13. Therefore, an aluminum atom has fourteen neutrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Determine the number of protons and. Why is aluminum in period 3? Hence, it lies in group 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons It has an atomic weight of 26.98154 and a mass. How many protons, neutrons, and electrons does aluminum contain? It is the only primordial aluminium isotope, i.e. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Aluminum (al) has an atomic. Aluminum Atom Protons Neutrons Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Protons Neutrons Electrons Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. How many protons, neutrons, and electrons does aluminum contain? Why is aluminum in period 3? 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Hence, it lies. Aluminum Atom Protons Neutrons Electrons.
From valenceelectrons.com
How many protons, neutrons and electrons does nickel have? Aluminum Atom Protons Neutrons Electrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum (al) has an atomic number of 13, which means it has 13. It has an atomic weight of 26.98154 and a mass. Determine the number of protons and. Why is aluminum in period 3? This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Therefore, an. Aluminum Atom Protons Neutrons Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Protons Neutrons Electrons Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. How many protons, neutrons, and electrons does aluminum contain? 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum. Aluminum Atom Protons Neutrons Electrons.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminum Atom Protons Neutrons Electrons Why is aluminum in period 3? Describe the locations, charges, and masses of the three main subatomic particles. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 112 rows protons, neutrons and electrons of all elements are mentioned in the table. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons Aluminum (al) has an atomic number of 13, which means it has 13. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. It has an atomic weight of 26.98154 and a mass. Describe the locations, charges, and masses of the three main subatomic particles. The difference between the mass. Aluminum Atom Protons Neutrons Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Therefore, an aluminum atom has fourteen neutrons. The difference between the mass number of the aluminum atom and the number of protons is fourteen. Aluminum (al) has an atomic number of 13, which means it has 13. Why. Aluminum Atom Protons Neutrons Electrons.
From awesomehome.co
Using The Periodic Table To Determine Protons Neutrons And Electrons Aluminum Atom Protons Neutrons Electrons Aluminum (al) has an atomic number of 13, which means it has 13. Determine the number of protons and. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. It is the only. Aluminum Atom Protons Neutrons Electrons.
From www.youtube.com
How many protons, neutrons, and electrons are present in an atom of Aluminum Atom Protons Neutrons Electrons How many protons, neutrons, and electrons does aluminum contain? Describe the locations, charges, and masses of the three main subatomic particles. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Hence, it lies in group 13. Determine the number of protons and. It has an atomic weight of 26.98154 and a mass. It is. Aluminum Atom Protons Neutrons Electrons.
From sciencepediablog.wordpress.com
Atoms (electron, neutron and protons) Sciencepedia Aluminum Atom Protons Neutrons Electrons Therefore, an aluminum atom has fourteen neutrons. Aluminum (al) has an atomic number of 13, which means it has 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. How many protons, neutrons, and electrons does aluminum contain? 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum. Aluminum Atom Protons Neutrons Electrons.
From sites.google.com
Structure of the Atom Unit G Review Aluminum Atom Protons Neutrons Electrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Determine the number of protons and. Therefore, an aluminum atom has fourteen neutrons. Describe the locations, charges, and masses of the three main subatomic particles. The difference between the mass number of. Aluminum Atom Protons Neutrons Electrons.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminum Atom Protons Neutrons Electrons Hence, it lies in group 13. Therefore, an aluminum atom has fourteen neutrons. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum (al) has an atomic number of 13, which means. Aluminum Atom Protons Neutrons Electrons.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation, free download ID Aluminum Atom Protons Neutrons Electrons Describe the locations, charges, and masses of the three main subatomic particles. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Therefore, an aluminum atom has fourteen neutrons. Aluminum (al) has an atomic number of 13, which means it has 13. Determine the number of protons and. The difference between the mass number of. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons Hence, it lies in group 13. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum (al) has an atomic number of 13, which means it has 13. How many protons, neutrons, and electrons does aluminum contain? Therefore, an aluminum atom has fourteen neutrons. Describe the locations,. Aluminum Atom Protons Neutrons Electrons.
From stock.adobe.com
Diagram of an oxygen atom with nucleus and inner and outer shells Aluminum Atom Protons Neutrons Electrons Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Therefore, an aluminum atom has fourteen neutrons. It has an atomic weight of 26.98154 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Describe the locations, charges, and masses of the three main subatomic particles. Aluminum (al) has. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum (al) has an atomic number of 13, which means it has 13. Hence, it lies in group 13. The difference between the mass number of the aluminum atom and the number of protons is fourteen. Describe the. Aluminum Atom Protons Neutrons Electrons.
From www.museoinclusivo.com
Exploring Aluminum Protons Neutrons Electrons An Indepth Look Aluminum Atom Protons Neutrons Electrons Determine the number of protons and. It has an atomic weight of 26.98154 and a mass. It is the only primordial aluminium isotope, i.e. Hence, it lies in group 13. The difference between the mass number of the aluminum atom and the number of protons is fourteen. This electron arrangement indicates that the outermost orbit of aluminum element (al) has. Aluminum Atom Protons Neutrons Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Protons Neutrons Electrons How many protons, neutrons, and electrons does aluminum contain? 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of. Aluminum Atom Protons Neutrons Electrons.
From www.teachoo.com
Neutron Discovery, Difference and more Teachoo Concepts Aluminum Atom Protons Neutrons Electrons Determine the number of protons and. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Why is aluminum in period 3? How many protons, neutrons, and electrons does aluminum contain? The difference between the. Aluminum Atom Protons Neutrons Electrons.
From www.numerade.com
SOLVED 'I need help filling out nitrogen Aluminum atom Aluminum ion Aluminum Atom Protons Neutrons Electrons Why is aluminum in period 3? The difference between the mass number of the aluminum atom and the number of protons is fourteen. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. It has an atomic weight of 26.98154 and a mass. Aluminum (al) has an atomic number of. Aluminum Atom Protons Neutrons Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons And Electrons Elcho Table Aluminum Atom Protons Neutrons Electrons This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. It has an atomic weight of 26.98154 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. How many protons, neutrons, and electrons does aluminum contain? Therefore, an aluminum atom. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Aluminum (al) has an atomic number of 13, which means it has 13. The difference between the mass number of the aluminum atom and the number of protons is fourteen. It has an atomic weight of 26.98154 and a mass.. Aluminum Atom Protons Neutrons Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Protons Neutrons Electrons Aluminum (al) has an atomic number of 13, which means it has 13. Determine the number of protons and. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Therefore, an aluminum atom has fourteen neutrons. Hence, it lies in group 13.. Aluminum Atom Protons Neutrons Electrons.
From ar.inspiredpencil.com
Aluminum Atom Model Aluminum Atom Protons Neutrons Electrons Hence, it lies in group 13. It has an atomic weight of 26.98154 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. How many. Aluminum Atom Protons Neutrons Electrons.
From www.animalia-life.club
Electrons In An Atom Aluminum Atom Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. It is the only primordial aluminium isotope, i.e. Describe the locations, charges, and masses of the three main subatomic particles. Why is aluminum in period 3? This electron arrangement indicates that the outermost orbit of aluminum element (al). Aluminum Atom Protons Neutrons Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Protons Neutrons Electrons It is the only primordial aluminium isotope, i.e. Determine the number of protons and. Aluminum (al) has an atomic number of 13, which means it has 13. It has an atomic weight of 26.98154 and a mass. Why is aluminum in period 3? Hence, it lies in group 13. How many protons, neutrons, and electrons does aluminum contain? The difference. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons It is the only primordial aluminium isotope, i.e. It has an atomic weight of 26.98154 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13.. Aluminum Atom Protons Neutrons Electrons.
From rokanggun.blogspot.com
18+ Aluminum Atome Aluminum Atom Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum (al) has an atomic number of 13, which means it has 13. The difference between the mass number of the aluminum atom and the number of protons is fourteen. Therefore, an aluminum atom has fourteen neutrons. Describe. Aluminum Atom Protons Neutrons Electrons.
From aluminumgenjin.blogspot.com
Aluminum Electron Configuration For Aluminum Aluminum Atom Protons Neutrons Electrons The difference between the mass number of the aluminum atom and the number of protons is fourteen. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons.. Aluminum Atom Protons Neutrons Electrons.
From finchsque1992.blogspot.com
W Number Of Protons Calculate The Number Of Protons Electrons And Aluminum Atom Protons Neutrons Electrons Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is the only primordial aluminium isotope, i.e. Determine the number of protons and. Why is aluminum in period 3? Therefore, an aluminum atom has fourteen neutrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. How many protons,. Aluminum Atom Protons Neutrons Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Protons Neutrons Electrons Hence, it lies in group 13. Aluminum (al) has an atomic number of 13, which means it has 13. Therefore, an aluminum atom has fourteen neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. Determine the number of protons and. The difference between the mass. Aluminum Atom Protons Neutrons Electrons.
From www.dreamstime.com
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminum Atom Protons Neutrons Electrons How many protons, neutrons, and electrons does aluminum contain? Why is aluminum in period 3? Therefore, an aluminum atom has fourteen neutrons. Aluminum (al) has an atomic number of 13, which means it has 13. Describe the locations, charges, and masses of the three main subatomic particles. The difference between the mass number of the aluminum atom and the number. Aluminum Atom Protons Neutrons Electrons.
From mungfali.com
10 Protons 11 Neutrons 10 Electrons Aluminum Atom Protons Neutrons Electrons How many protons, neutrons, and electrons does aluminum contain? Describe the locations, charges, and masses of the three main subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Aluminum (al) has an atomic number of 13, which means it has 13. Hence, it lies in. Aluminum Atom Protons Neutrons Electrons.
From ask.modifiyegaraj.com
How Many Neutrons Does Aluminum Have Asking List Aluminum Atom Protons Neutrons Electrons How many protons, neutrons, and electrons does aluminum contain? Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Therefore, an aluminum atom has fourteen neutrons. It has an atomic weight of 26.98154 and a mass. The difference between the mass number of the aluminum atom and the number of protons is fourteen. Why is aluminum in period 3? Determine the number. Aluminum Atom Protons Neutrons Electrons.
From www.numerade.com
SOLVED 'help me with this please 10. What do the numbers 13 and 27 Aluminum Atom Protons Neutrons Electrons This electron arrangement indicates that the outermost orbit of aluminum element (al) has 3 electrons. It is the only primordial aluminium isotope, i.e. Aluminum (al) has an atomic number of 13, which means it has 13. Determine the number of protons and. How many protons, neutrons, and electrons does aluminum contain? Therefore, an aluminum atom has fourteen neutrons. 112 rows. Aluminum Atom Protons Neutrons Electrons.