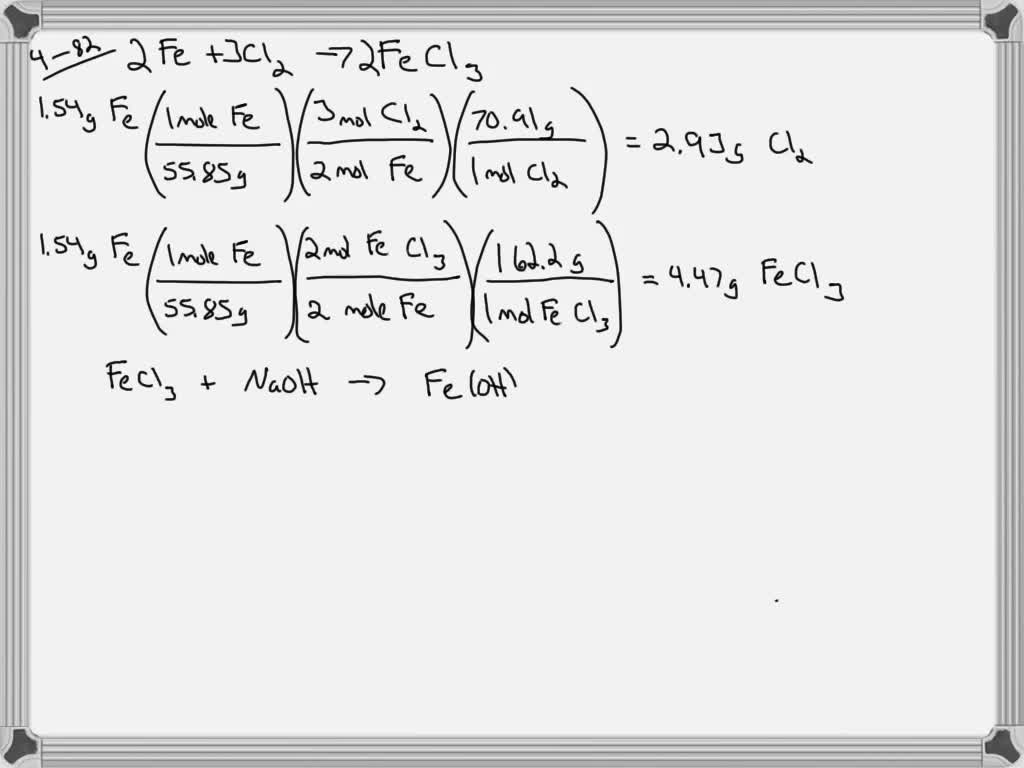
Iron Iii Chloride Reacts With Sodium Hydroxide . The iron(iii)hydroxide is probably an. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The result is a redox reaction where fe 3+ oxidizes i. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). Adding sodium hydroxide solution dropwise to fecl 3. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium hydroxide. Reaction of sodium hydroxide solution with iron (iii) chloride solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. The reaction of chlorine with hot sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and.
from www.numerade.com
A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Iron (iii) ion with sodium iodide. The result is a redox reaction where fe 3+ oxidizes i. You can use ammonia solution instead of sodium hydroxide. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Adding sodium hydroxide solution dropwise to fecl 3. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction of chlorine with hot sodium hydroxide solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+).
SOLVEDIron(III) chloride is produced by the reaction of iron and
Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Iron (iii) ion with sodium iodide. Reaction of sodium hydroxide solution with iron (iii) chloride solution. The iron(iii)hydroxide is probably an. The result is a redox reaction where fe 3+ oxidizes i. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. The reaction of chlorine with hot sodium hydroxide solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). Adding sodium hydroxide solution dropwise to fecl 3.
From www.numerade.com
What mass of iron(III) hydroxide precipitate can be produced by Iron Iii Chloride Reacts With Sodium Hydroxide Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium hydroxide. The iron(iii)hydroxide is probably an. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). Adding sodium hydroxide solution dropwise to. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. The reaction of chlorine with hot sodium hydroxide solution. Adding sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation ID5348558 Iron Iii Chloride Reacts With Sodium Hydroxide Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The iron(iii)hydroxide is probably an. Iron (iii) ion with sodium iodide. The result is a redox reaction where fe 3+ oxidizes i. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction of chlorine with hot sodium hydroxide solution.. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Iron (III) chloride (FeCl3) with Ammonium hydroxide (NH4OH Iron Iii Chloride Reacts With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. The result is a redox reaction where fe 3+ oxidizes i. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Iron (iii) ion with sodium iodide. Adding sodium hydroxide solution dropwise to fecl 3. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. A solution of iron(iii)chloride is. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Iron Iii Chloride Reacts With Sodium Hydroxide Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The reaction of chlorine with hot sodium hydroxide solution. The result is a redox reaction where fe 3+ oxidizes i. You can use ammonia solution instead of sodium hydroxide. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Reaction of sodium hydroxide solution with iron (iii). Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.alamy.com
Iron (III) chloride (FeCl3) solution. When sodium hydroxide is added to Iron Iii Chloride Reacts With Sodium Hydroxide Reaction of sodium hydroxide solution with iron (iii) chloride solution. Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium hydroxide. The iron(iii)hydroxide is probably an. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The reaction of chlorine with hot sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution. Iron Iii Chloride Reacts With Sodium Hydroxide.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction of chlorine with hot sodium hydroxide solution. Iron (iii) ion with sodium iodide. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The iron(iii)hydroxide is probably an. The. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Adding sodium hydroxide solution dropwise to fecl 3. Reaction of sodium hydroxide solution with iron (iii) chloride solution. The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Sodium hydroxide and iron III chloride Stock Image C028/4185 Iron Iii Chloride Reacts With Sodium Hydroxide Adding sodium hydroxide solution dropwise to fecl 3. The iron(iii)hydroxide is probably an. You can use ammonia solution instead of sodium hydroxide. The reaction of chlorine with hot sodium hydroxide solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+).. Iron Iii Chloride Reacts With Sodium Hydroxide.
From brainly.com
Iron (2) Chloride reacts with sodium oxide to form...? Iron Iii Chloride Reacts With Sodium Hydroxide Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. The reaction of chlorine with hot sodium hydroxide solution. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. Iron (iii) ion with sodium iodide. You. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Iron(III) Nitrate (Fe(NO3)3) and Sodium hydroxide (NaOH Iron Iii Chloride Reacts With Sodium Hydroxide The reaction of chlorine with hot sodium hydroxide solution. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Adding sodium hydroxide solution dropwise to fecl 3. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The reaction between chlorine and hot concentrated sodium hydroxide solution is:. The iron(iii)hydroxide is probably an. The result is a redox. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Iron (II) Sulphate (FeSO4) with Sodium Hydroxide (NaOH Iron Iii Chloride Reacts With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. Reaction of sodium hydroxide solution with iron (iii) chloride solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with sodium iodide. The iron(iii)hydroxide is probably an. Here, sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.alamy.com
Iron (III) hydroxide precipitation. Test tube containing sodium Iron Iii Chloride Reacts With Sodium Hydroxide Reaction of sodium hydroxide solution with iron (iii) chloride solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). You can use ammonia solution instead of sodium hydroxide. Iron (iii) ion with sodium iodide. The reaction of chlorine with hot sodium hydroxide solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Adding sodium hydroxide. Iron Iii Chloride Reacts With Sodium Hydroxide.
From stock.adobe.com
Chemical reactionRusty red iron(III) hydroxide precipitate (Fe(OH)3 Iron Iii Chloride Reacts With Sodium Hydroxide The reaction of chlorine with hot sodium hydroxide solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. Adding sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron Iii Chloride Reacts With Sodium Hydroxide Adding sodium hydroxide solution dropwise to fecl 3. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. The result is a redox reaction where fe 3+. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.slideserve.com
PPT Preview PowerPoint Presentation, free download ID6531641 Iron Iii Chloride Reacts With Sodium Hydroxide The reaction between chlorine and hot concentrated sodium hydroxide solution is:. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. Iron (iii) ion with sodium iodide. You can use ammonia solution. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.toppr.com
When chlorine reacts with cold and dilute solution of sodium hydroxide Iron Iii Chloride Reacts With Sodium Hydroxide Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Reaction of sodium hydroxide solution with iron (iii) chloride solution. The iron(iii)hydroxide is probably an. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. Adding sodium hydroxide solution dropwise to fecl 3. The reaction of. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image C027/9448 Science Iron Iii Chloride Reacts With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. Iron (iii) ion with sodium iodide. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Adding sodium hydroxide solution dropwise to fecl 3. A solution of iron(iii)chloride. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Addition sodium hydroxide to iron (II) chloride and iron (III) chloride Iron Iii Chloride Reacts With Sodium Hydroxide Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. The iron(iii)hydroxide is probably an. Adding sodium hydroxide solution dropwise to fecl 3. Reaction of sodium hydroxide solution with iron (iii) chloride solution. The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with. Iron Iii Chloride Reacts With Sodium Hydroxide.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. The iron(iii)hydroxide is probably an. Iron (iii) ion with sodium iodide. The reaction between chlorine and hot concentrated sodium hydroxide solution is:.. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
The reaction of iron(III) solution with sodium hydroxide and ammonia Iron Iii Chloride Reacts With Sodium Hydroxide The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Adding sodium hydroxide solution dropwise to fecl 3. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). You can use ammonia solution instead of sodium hydroxide. The reaction of chlorine with hot sodium hydroxide solution. A solution of iron(iii)chloride is mixed with a solution of sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride Reacts With Sodium Hydroxide Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium hydroxide. The iron(iii)hydroxide is probably an. Adding sodium hydroxide solution dropwise to fecl 3. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The result is a redox reaction where fe 3+ oxidizes i. A solution of iron(iii)chloride is mixed with a solution of. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Iron Iii Chloride Reacts With Sodium Hydroxide Iron (iii) ion with sodium iodide. The result is a redox reaction where fe 3+ oxidizes i. You can use ammonia solution instead of sodium hydroxide. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Adding sodium hydroxide solution dropwise to fecl 3. The iron(iii)hydroxide is probably an. 7 rows solutions containing copper (ii) ions form a blue precipitate. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide Iron Iii Chloride Reacts With Sodium Hydroxide The result is a redox reaction where fe 3+ oxidizes i. The iron(iii)hydroxide is probably an. Iron (iii) ion with sodium iodide. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Reaction of sodium hydroxide solution with. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
The Reaction Between Iron (III) Nitrate and Sodium Hydroxide YouTube Iron Iii Chloride Reacts With Sodium Hydroxide The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with sodium iodide. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). Adding sodium hydroxide solution dropwise to fecl 3. Reaction of sodium hydroxide solution with iron (iii) chloride solution. 7 rows. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVEDIron(III) chloride is produced by the reaction of iron and Iron Iii Chloride Reacts With Sodium Hydroxide The reaction between chlorine and hot concentrated sodium hydroxide solution is:. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The reaction of chlorine with hot sodium hydroxide solution. The result is a redox reaction where fe 3+ oxidizes i. Here,. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVEDIn water, iron(III) chloride reacts with sodium hydroxide Iron Iii Chloride Reacts With Sodium Hydroxide A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. You can use ammonia solution instead of sodium hydroxide. The reaction. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.alamy.com
Sodium hydroxide and iron III chloride. Test tube containing sodium Iron Iii Chloride Reacts With Sodium Hydroxide The result is a redox reaction where fe 3+ oxidizes i. Reaction of sodium hydroxide solution with iron (iii) chloride solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. A solution of iron(iii)chloride is mixed with a solution of sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
FeCl3+AgNO3=AgCl+Fe(NO3)3 Balanced EquationIron(iii)chloride+Silver Iron Iii Chloride Reacts With Sodium Hydroxide A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Adding sodium hydroxide solution dropwise to fecl 3. The reaction of chlorine with hot sodium hydroxide solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are. Iron Iii Chloride Reacts With Sodium Hydroxide.
From stock.adobe.com
Chemical reactionPipette 0.25 M solution of iron(III) chloride (FeCl3 Iron Iii Chloride Reacts With Sodium Hydroxide The result is a redox reaction where fe 3+ oxidizes i. Reaction of sodium hydroxide solution with iron (iii) chloride solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Adding sodium hydroxide solution dropwise to fecl 3. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Fecl_3(aq) + 3naoh(aq). Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.youtube.com
How to Balance NaOH + FeCl3 = Fe(OH)3 + NaCl Sodium hydroxide + Iron Iron Iii Chloride Reacts With Sodium Hydroxide Iron (iii) ion with sodium iodide. The iron(iii)hydroxide is probably an. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction of chlorine with hot sodium hydroxide solution. The result is a redox reaction where fe 3+ oxidizes i. Adding sodium hydroxide solution dropwise to fecl 3. Reaction of sodium hydroxide. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.chegg.com
Solved Part A Enter a balanced chemical equation for the Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The result is a redox reaction where fe 3+ oxidizes i. Iron (iii) ion with sodium iodide. A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. The reaction of chlorine with. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Iron Iii Chloride Reacts With Sodium Hydroxide The iron(iii)hydroxide is probably an. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are insoluble. The result is a redox reaction where fe 3+ oxidizes i. Adding sodium hydroxide solution dropwise to fecl 3. The reaction of chlorine with hot sodium hydroxide solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Iron (iii) ion with sodium. Iron Iii Chloride Reacts With Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride Reacts With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Here, sodium iodide (nai) is added to iron (iii) ion (fe 3+). A solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii) hydroxide and. Fecl_3(aq) + 3naoh(aq) rarr fe(oh)_3(s)darr + 3nacl(aq) most hydroxides are. Iron Iii Chloride Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Part 3 Aqueous potassium hydroxide reacts with aqueous Iron Iii Chloride Reacts With Sodium Hydroxide Iron (iii) ion with sodium iodide. You can use ammonia solution instead of sodium hydroxide. The reaction of chlorine with hot sodium hydroxide solution. The reaction between chlorine and hot concentrated sodium hydroxide solution is:. Reaction of sodium hydroxide solution with iron (iii) chloride solution. Adding sodium hydroxide solution dropwise to fecl 3. Here, sodium iodide (nai) is added to. Iron Iii Chloride Reacts With Sodium Hydroxide.