Chemistry Of Catalytic Converters . What is a catalytic converter? Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. That all sounds very dangerous, doesn’t it? The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. Fortunately, catalytic converters help make engine emissions less harmful. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They usually consist of a mixture of finely divided platinum and. Catalytic converters are used in car exhaust boxes to reduce air pollution. We are required to deduce the reactions occurring in the catalytic converter.
from scitechdaily.com
That all sounds very dangerous, doesn’t it? What is a catalytic converter? Catalytic converters are used in car exhaust boxes to reduce air pollution. They contain a series of transition metal catalysts including platinum and rhodium. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They usually consist of a mixture of finely divided platinum and. Fortunately, catalytic converters help make engine emissions less harmful. We are required to deduce the reactions occurring in the catalytic converter. The metal catalysts are in a. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter.
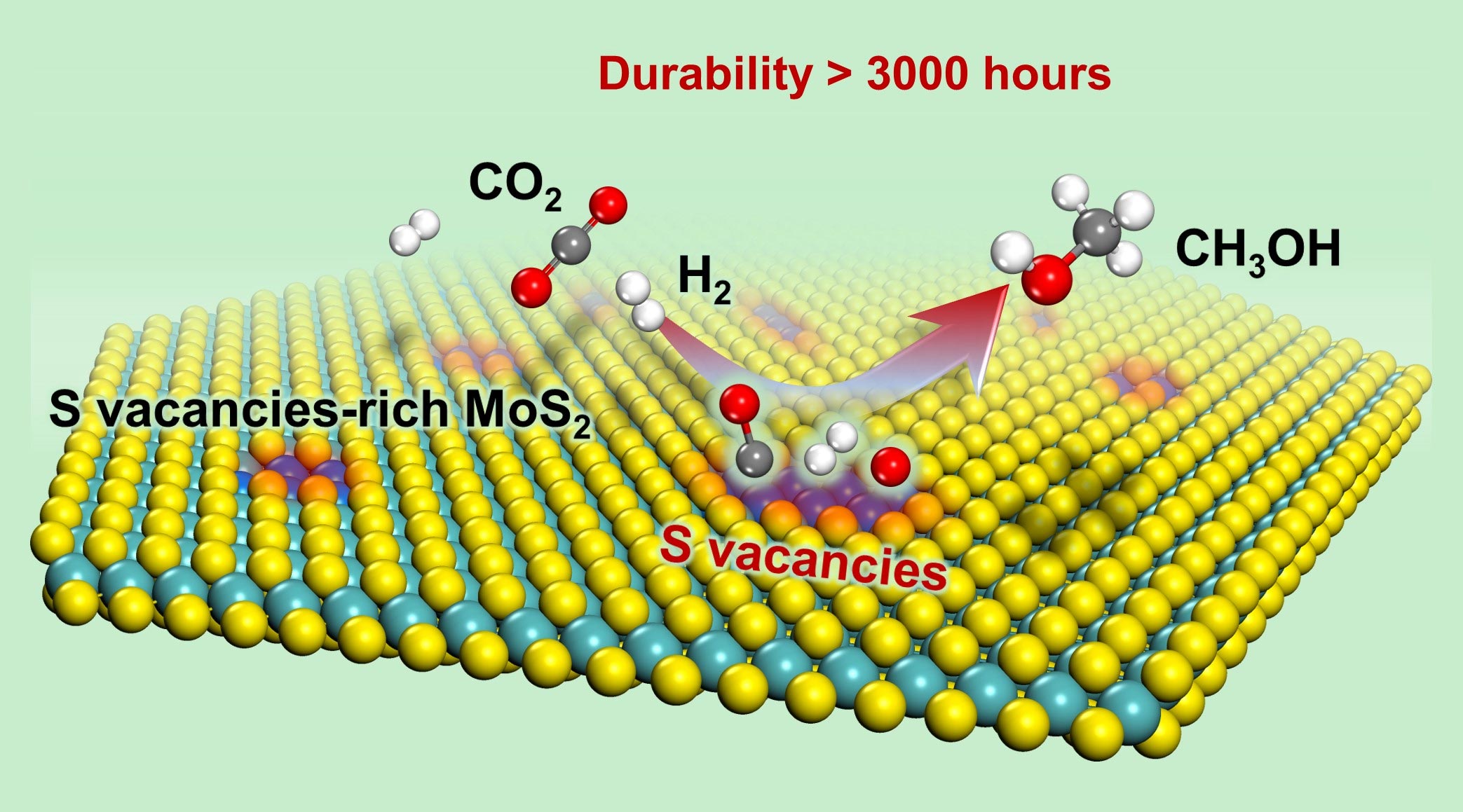
Catalytic Hydrogenation of CO2 to Methanol Low Temperature and High
Chemistry Of Catalytic Converters What is a catalytic converter? Fortunately, catalytic converters help make engine emissions less harmful. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust boxes to reduce air pollution. They contain a series of transition metal catalysts including platinum and rhodium. We are required to deduce the reactions occurring in the catalytic converter. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. The metal catalysts are in a. That all sounds very dangerous, doesn’t it? What is a catalytic converter? They usually consist of a mixture of finely divided platinum and. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.2.10 Heterogeneous Catalysis翰林国际教育 Chemistry Of Catalytic Converters The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. What is a catalytic converter? Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters. Chemistry Of Catalytic Converters.
From engineeringlearner.com
What is Catalytic Converter? Engineering Learner Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? The metal catalysts are in a. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They usually consist of a mixture of finely divided platinum and. We are required to deduce the reactions occurring in the catalytic converter. What is. Chemistry Of Catalytic Converters.
From www.ecotradegroup.com
Ecotrade Group What is a catalytic converter? Chemistry Of Catalytic Converters Catalytic converters are used in car exhaust boxes to reduce air pollution. We are required to deduce the reactions occurring in the catalytic converter. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon. Chemistry Of Catalytic Converters.
From gcse.fandom.com
Catalytic Converters GCSE Wiki Fandom Chemistry Of Catalytic Converters Fortunately, catalytic converters help make engine emissions less harmful. They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They contain a series of transition metal catalysts including platinum and rhodium. What is a catalytic converter? Use. Chemistry Of Catalytic Converters.
From www.issuewire.com
New Patented App Tracks Warranties of Catalytic Converters IssueWire Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. What is a catalytic converter? That all sounds very dangerous, doesn’t it? The metal catalysts are in a. Here is what would happen when pollutants from exhaust fumes. Chemistry Of Catalytic Converters.
From hdpmotor.blogspot.com
how to work catalytic converter hdp motor Chemistry Of Catalytic Converters Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. Fortunately, catalytic converters help make engine emissions less harmful. That all sounds very dangerous, doesn’t it? They contain a series of transition metal catalysts including platinum and rhodium. What is a catalytic converter? They usually consist of a mixture of finely divided platinum and. Use. Chemistry Of Catalytic Converters.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.4.2 Nitrogen Oxides翰林国际教育 Chemistry Of Catalytic Converters Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. What is a catalytic converter? We are required to deduce the reactions occurring in the catalytic converter. Fortunately,. Chemistry Of Catalytic Converters.
From www.thinkswap.com
Chemistry 2.3 Catalytic Converters Excellence Exemplar Chemistry Chemistry Of Catalytic Converters What is a catalytic converter? They usually consist of a mixture of finely divided platinum and. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. We are required to deduce the reactions occurring in the catalytic converter. They contain a series of transition metal catalysts including platinum and rhodium.. Chemistry Of Catalytic Converters.
From www.youtube.com
How 3 way catalytic converters work by Howstuffinmycarworks YouTube Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. The metal catalysts are in a. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. Catalytic converters are used in. Chemistry Of Catalytic Converters.
From www.merityre.co.uk
Catalytic Converters Merityre Specialists Chemistry Of Catalytic Converters What is a catalytic converter? Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They usually consist of a mixture of finely divided platinum and. That all sounds very dangerous, doesn’t it? The metal catalysts are in a. Fortunately, catalytic converters help make engine emissions less harmful. We are. Chemistry Of Catalytic Converters.
From news.virginia.edu
University of Virginia Researchers Uncover New Catalysis Site UVA Today Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. Fortunately, catalytic converters help make engine emissions less harmful. The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. We are required to deduce the reactions occurring in the catalytic. Chemistry Of Catalytic Converters.
From www.youtube.com
What Are Catalytic Converters Chemistry for All FuseSchool YouTube Chemistry Of Catalytic Converters Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. The metal catalysts are in a. Catalytic converters are used in car exhaust boxes to reduce air pollution. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. Fortunately, catalytic converters help make. Chemistry Of Catalytic Converters.
From tipking.com
How to Clean Catalytic Converter without Removing it TipKing Chemistry Of Catalytic Converters The metal catalysts are in a. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. That all sounds very dangerous, doesn’t it? We are required to deduce. Chemistry Of Catalytic Converters.
From knowhow.napaonline.com
How A Catalytic Converter Works » NAPA Blog Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? Fortunately, catalytic converters help make engine emissions less harmful. We are required to deduce the reactions occurring in the catalytic converter. What is a catalytic converter? The metal catalysts are in a. They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various. Chemistry Of Catalytic Converters.
From www.mdpi.com
Chemistry Free FullText Catalytic Converters for Vehicle Exhaust Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. What is a catalytic converter? The metal catalysts are in a. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They usually consist of a mixture of finely divided platinum and. We are required to deduce the. Chemistry Of Catalytic Converters.
From www.hidenanalytical.com
New Hiden Catalysis Applications Catalogue Hiden Analytical Chemistry Of Catalytic Converters Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. Fortunately, catalytic converters help make engine emissions less harmful. Catalytic converters are used in car exhaust boxes to reduce air pollution. We are required to deduce the. Chemistry Of Catalytic Converters.
From chemrxiv.org
Catalytic converters Enhancing Green Chemistry and Their Technological Chemistry Of Catalytic Converters Catalytic converters are used in car exhaust boxes to reduce air pollution. They usually consist of a mixture of finely divided platinum and. They contain a series of transition metal catalysts including platinum and rhodium. Fortunately, catalytic converters help make engine emissions less harmful. We are required to deduce the reactions occurring in the catalytic converter. Here is what would. Chemistry Of Catalytic Converters.
From www.mdpi.com
Chemistry Free FullText Catalytic Converters for Vehicle Exhaust Chemistry Of Catalytic Converters They usually consist of a mixture of finely divided platinum and. Fortunately, catalytic converters help make engine emissions less harmful. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. What is a catalytic converter? They contain a series of transition metal catalysts including platinum and rhodium. Use. Chemistry Of Catalytic Converters.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. Use our revision notes to understand catalytic converters for your a level chemistry. Chemistry Of Catalytic Converters.
From crunchchemistry.co.uk
Heterogeneous catalysis and catalytic converters Crunch Chemistry Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. What is a catalytic converter? Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more. Chemistry Of Catalytic Converters.
From chem.libretexts.org
12.8 Catalysis Chemistry LibreTexts Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? They contain a series of transition metal catalysts including platinum and rhodium. Catalytic converters are used in car exhaust boxes to reduce air pollution. They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless. Chemistry Of Catalytic Converters.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Chemistry Of Catalytic Converters We are required to deduce the reactions occurring in the catalytic converter. Catalytic converters are used in car exhaust boxes to reduce air pollution. They contain a series of transition metal catalysts including platinum and rhodium. What is a catalytic converter? Fortunately, catalytic converters help make engine emissions less harmful. Catalytic converters change poisonous molecules like carbon monoxide and various. Chemistry Of Catalytic Converters.
From scitechdaily.com
Catalytic Hydrogenation of CO2 to Methanol Low Temperature and High Chemistry Of Catalytic Converters That all sounds very dangerous, doesn’t it? The metal catalysts are in a. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They usually consist of a mixture of finely divided platinum and. Here is what would happen when pollutants from exhaust fumes pass through the catalytic. Chemistry Of Catalytic Converters.
From artfer.net
Benefits of Buying Catalytic Converters Artfer Chemistry Of Catalytic Converters The metal catalysts are in a. Catalytic converters are used in car exhaust boxes to reduce air pollution. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide.. Chemistry Of Catalytic Converters.
From instagrid.me
The Science Behind Catalytic Converters and Their Role in Reducing Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. We are required to deduce the reactions occurring in the catalytic converter. They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. Fortunately, catalytic converters. Chemistry Of Catalytic Converters.
From www.researchgate.net
Installation of different types of catalytic converter systems. Adapted Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. That all sounds very dangerous, doesn’t it? Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust boxes to reduce air pollution. We are required to deduce the reactions occurring in. Chemistry Of Catalytic Converters.
From www.youtube.com
What Are Catalytic Converters Environment Chemistry FuseSchool Chemistry Of Catalytic Converters They contain a series of transition metal catalysts including platinum and rhodium. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust boxes to reduce air pollution. The metal catalysts are in a. Fortunately, catalytic converters help make engine emissions less harmful. Here. Chemistry Of Catalytic Converters.
From innovationdiscoveries.space
Catalytic Converter (3way) Chemistry Of Catalytic Converters We are required to deduce the reactions occurring in the catalytic converter. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. What is a catalytic converter? They contain a series of transition metal catalysts including platinum and rhodium. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into. Chemistry Of Catalytic Converters.
From www.brighthub.com
Answers to, How Are Catalytic Converters Recycled Chemistry Of Catalytic Converters Catalytic converters are used in car exhaust boxes to reduce air pollution. What is a catalytic converter? That all sounds very dangerous, doesn’t it? They usually consist of a mixture of finely divided platinum and. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They contain a series of. Chemistry Of Catalytic Converters.
From poddtoppen.se
What do catalytic converters do? Chemistry For Your Life Lyssna här Chemistry Of Catalytic Converters Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. The metal catalysts are in a. We are required to deduce the reactions occurring in the catalytic converter. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust. Chemistry Of Catalytic Converters.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Chemistry Of Catalytic Converters We are required to deduce the reactions occurring in the catalytic converter. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. They usually consist of a mixture of finely divided platinum and. Catalytic converters are used in car exhaust boxes to reduce air pollution. Fortunately, catalytic converters. Chemistry Of Catalytic Converters.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Chemistry Of Catalytic Converters We are required to deduce the reactions occurring in the catalytic converter. That all sounds very dangerous, doesn’t it? The metal catalysts are in a. They contain a series of transition metal catalysts including platinum and rhodium. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Fortunately, catalytic converters. Chemistry Of Catalytic Converters.
From present5.com
Brief History of the Catalytic Converter The catalytic Chemistry Of Catalytic Converters Fortunately, catalytic converters help make engine emissions less harmful. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust boxes to reduce air pollution. We are required to deduce the reactions occurring in the catalytic converter. They usually consist of a mixture of. Chemistry Of Catalytic Converters.
From www.youtube.com
Chemistry Chemical (29 of 30) How Does the Catalytic Chemistry Of Catalytic Converters They usually consist of a mixture of finely divided platinum and. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide. Here is what would happen when pollutants from exhaust fumes pass through the catalytic converter. We are required to deduce the reactions occurring in the catalytic converter.. Chemistry Of Catalytic Converters.
From www.behance.net
Catalytic Converter Vector Illustration Behance Chemistry Of Catalytic Converters We are required to deduce the reactions occurring in the catalytic converter. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalytic converters are used in car exhaust boxes to reduce air pollution. They contain a series of transition metal catalysts including platinum and rhodium. Fortunately, catalytic converters help. Chemistry Of Catalytic Converters.