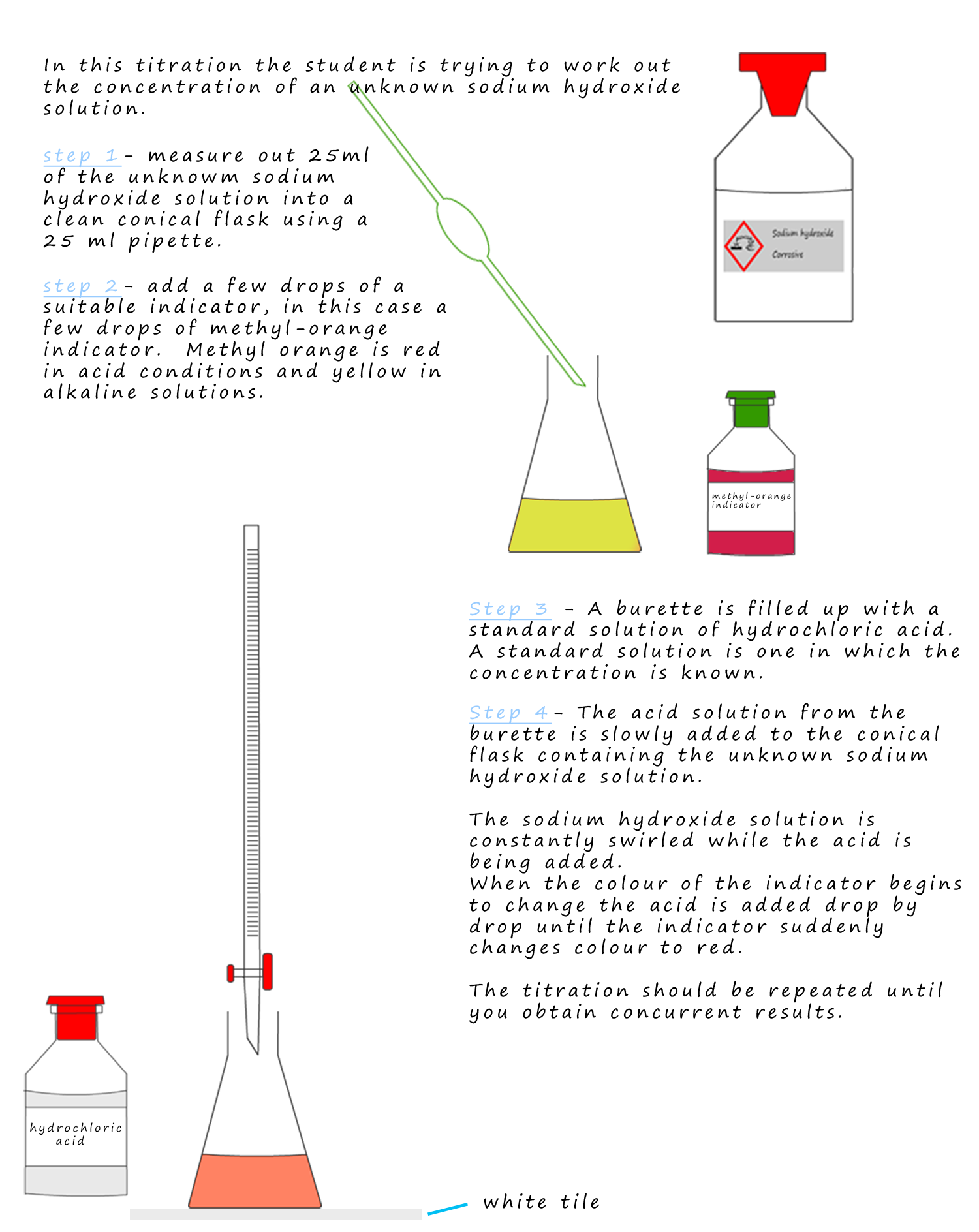
Titration To Find Concentration . By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. Suppose that a titration is performed and \(20.70 \: Concentration = moles ÷ volume (in dm³). The concentration of an unknown solution is found using the relationship: When the reaction between the. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point when titrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly.
from www.science-revision.co.uk
Titration calculations are used to find the concentration of unknown solutions. Concentration = moles ÷ volume (in dm³). In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. When the reaction between the. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Suppose that a titration is performed and \(20.70 \: The concentration of an unknown solution is found using the relationship:
Titrations
Titration To Find Concentration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The concentration of an unknown solution is found using the relationship: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. They can also be used to calculate the ph after a given point during a titration. Concentration = moles ÷ volume (in dm³). \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Suppose that a titration is performed and \(20.70 \: Titration calculations are used to find the concentration of unknown solutions. When the reaction between the. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew.
From hxenjakty.blob.core.windows.net
Titration Types Definition at John Justice blog Titration To Find Concentration The basic process involves adding a. Suppose that a titration is performed and \(20.70 \: Concentration = moles ÷ volume (in dm³). Titration calculations are used to find the concentration of unknown solutions. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end. Titration To Find Concentration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration To Find Concentration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. \ce{naoh}\) is required to reach the end point when titrated. When the reaction between the. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. Titration To Find Concentration.
From www.numerade.com
SOLVED EXPERIMENT 3ACID BASE TITRATIONS NEUTRALIZATION REACTION Aim Titration To Find Concentration When the reaction between the. They can also be used to calculate the ph after a given point during a titration. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. A titration is a laboratory technique used to precisely. Titration To Find Concentration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration To Find Concentration When the reaction between the. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a. They can also be used to calculate the ph after a given point during a titration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The concentration of an. Titration To Find Concentration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration To Find Concentration They can also be used to calculate the ph after a given point during a titration. Suppose that a titration is performed and \(20.70 \: When the reaction between the. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew.. Titration To Find Concentration.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Titration To Find Concentration They can also be used to calculate the ph after a given point during a titration. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. \ce{naoh}\) is required to reach the end point when titrated. A titration is a. Titration To Find Concentration.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration To Find Concentration The basic process involves adding a. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Concentration = moles. Titration To Find Concentration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration To Find Concentration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). They can also be used to calculate the ph after a given point during a titration. The basic process involves adding a. Concentration = moles ÷ volume (in dm³). Titration calculations are used to find the concentration of unknown solutions.. Titration To Find Concentration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The concentration of an unknown solution is found using the relationship: They can also be used to calculate the ph after a given point during a titration. Titration is a method to determine the unknown concentration of a specific substance. Titration To Find Concentration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration To Find Concentration \ce{naoh}\) is required to reach the end point when titrated. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: Concentration = moles ÷ volume (in dm³). Titration calculations are used to find the concentration. Titration To Find Concentration.
From www.slideshare.net
Titration report Titration To Find Concentration By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. When the reaction between the. Let's assume you are titrating. Titration To Find Concentration.
From www.maieutapedia.org
Stoichiometry and Titration Maieuta Pedia Titration To Find Concentration Titration calculations are used to find the concentration of unknown solutions. Concentration = moles ÷ volume (in dm³). The basic process involves adding a. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory. Titration To Find Concentration.
From dokumen.tips
(PDF) Required Practical 1 Titrations AQA CHEM/AS Revision/AS... · 1 Titration To Find Concentration By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the. Let's assume you are titrating a strong acid (10 ml. Titration To Find Concentration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration To Find Concentration Titration calculations are used to find the concentration of unknown solutions. \ce{naoh}\) is required to reach the end point when titrated. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Concentration = moles ÷ volume (in dm³). The concentration. Titration To Find Concentration.
From mungfali.com
Acid Base Titration Calculation Titration To Find Concentration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. \ce{naoh}\) is required to reach the end point when titrated. By far the most common use of titrations is. Titration To Find Concentration.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration To Find Concentration When the reaction between the. The basic process involves adding a. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration To Find Concentration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration To Find Concentration The concentration of an unknown solution is found using the relationship: When the reaction between the. Concentration = moles ÷ volume (in dm³). Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point. Titration To Find Concentration.
From www.science-revision.co.uk
Titrations Titration To Find Concentration By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Titration calculations are used to find the concentration of unknown solutions. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is a method. Titration To Find Concentration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration To Find Concentration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration calculations are used to find. Titration To Find Concentration.
From www.chegg.com
Solved Experiment 11, Titrations Report Sheet Titration To Find Concentration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration To Find Concentration.
From theedge.com.hk
Chemistry How To Titration The Edge Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a. Concentration = moles ÷ volume (in dm³). \ce{naoh}\) is required to reach the end point when titrated. They can also be used to calculate the. Titration To Find Concentration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration To Find Concentration The concentration of an unknown solution is found using the relationship: The basic process involves adding a. Concentration = moles ÷ volume (in dm³). By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Let's assume you are titrating a. Titration To Find Concentration.
From hxedyezjh.blob.core.windows.net
Acid Base Indicators Titration at Julie Corson blog Titration To Find Concentration Concentration = moles ÷ volume (in dm³). Suppose that a titration is performed and \(20.70 \: They can also be used to calculate the ph after a given point during a titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration is a laboratory technique used. Titration To Find Concentration.
From yojkglpsgc.blogspot.com
How To Calculate Initial Concentration From Titration Curve Volume of Titration To Find Concentration By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the. A titration is a laboratory technique used to precisely measure. Titration To Find Concentration.
From saylordotorg.github.io
AcidBase Titrations Titration To Find Concentration \ce{naoh}\) is required to reach the end point when titrated. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially. Titration To Find Concentration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration To Find Concentration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). When the reaction between the. They can also be used to calculate the ph after a given point during a titration. The basic process involves adding a. Concentration = moles ÷ volume (in dm³). Titration is a method to determine. Titration To Find Concentration.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a. They can also be used to calculate the ph after a given point during a titration. Concentration = moles ÷ volume (in dm³). Titration is. Titration To Find Concentration.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration To Find Concentration When the reaction between the. Titration calculations are used to find the concentration of unknown solutions. Suppose that a titration is performed and \(20.70 \: The concentration of an unknown solution is found using the relationship: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. By far the most common use of titrations is. Titration To Find Concentration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution Titration To Find Concentration Titration calculations are used to find the concentration of unknown solutions. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The concentration of an unknown solution is found using the relationship: By far the most common use of titrations is in determining unknowns, that is, in determining the concentration. Titration To Find Concentration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration To Find Concentration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. Titration calculations are used to find the concentration of unknown solutions. The basic process involves. Titration To Find Concentration.
From www.alamy.com
Titration hires stock photography and images Alamy Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. The concentration of an unknown solution is found using the relationship: They can also be used to calculate the ph after a given point during a titration. When the reaction between the. Let's assume. Titration To Find Concentration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration To Find Concentration Titration calculations are used to find the concentration of unknown solutions. Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Concentration = moles ÷ volume (in dm³). In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution. Titration To Find Concentration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic Titration To Find Concentration \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. By far the. Titration To Find Concentration.
From www.savemyexams.com
AcidAlkali Titrations (2.6.3) Edexcel IGCSE Chemistry Revision Notes Titration To Find Concentration The concentration of an unknown solution is found using the relationship: By far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount of substance in a sample about which we initially knew. \ce{naoh}\) is required to reach the end point when titrated. In a titration, 25.0 cm 3 of 0.100 mol/dm. Titration To Find Concentration.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration To Find Concentration They can also be used to calculate the ph after a given point during a titration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when. Titration To Find Concentration.