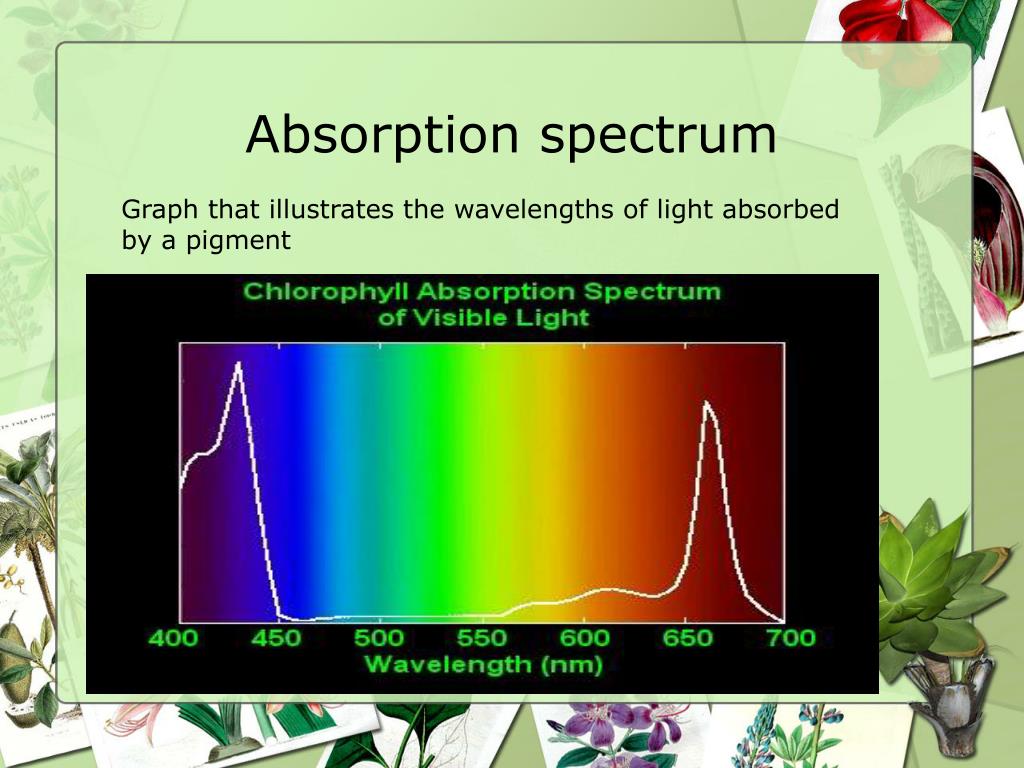
Absorption Line Spectrum Definition . You will know how spectral lines are. We can use a star’s absorption spectrum to figure out what elements it is made of. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. you will be able to distinguish between emission and absorption lines in a spectrum. let’s go back to simple absorption and emission spectra. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. (a) when a hydrogen atom absorbs.
from professionalsshery.weebly.com
an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. you will be able to distinguish between emission and absorption lines in a spectrum. let’s go back to simple absorption and emission spectra. (a) when a hydrogen atom absorbs. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. You will know how spectral lines are. We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of light by a hydrogen atom.
Color absorbance spectrum professionalsshery
Absorption Line Spectrum Definition an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. We can use a star’s absorption spectrum to figure out what elements it is made of. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. (a) when a hydrogen atom absorbs. let’s go back to simple absorption and emission spectra. You will know how spectral lines are. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. Absorption of light by a hydrogen atom.
From spacecentre.ca
Redshift H.R. MacMillan Space Centre Absorption Line Spectrum Definition an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. You will know how spectral lines are. We can use a star’s absorption spectrum to figure out what elements it is made of. . Absorption Line Spectrum Definition.
From www.slideserve.com
PPT “continuous” spectrum. PowerPoint Presentation, free download Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. let’s go back to simple absorption and emission spectra. you will be able to distinguish between emission and absorption lines in a spectrum. (a) when a hydrogen atom absorbs. We can use a star’s absorption spectrum to figure out what elements it is made of. You will know how spectral lines. Absorption Line Spectrum Definition.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Absorption Line Spectrum Definition You will know how spectral lines are. Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. We can use a star’s absorption spectrum to figure out what elements it is made of. let’s go back to simple absorption and emission spectra. (a) when. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Basics of Astronomy PowerPoint Presentation, free download ID Absorption Line Spectrum Definition an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. We can use a star’s absorption spectrum to figure out what elements it is made of. You will know how spectral lines are. let’s go back to simple absorption and emission spectra. the absorption spectrum is a spectrum of electromagnetic. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT WHAT DO YOU THINK? PowerPoint Presentation, free download ID301012 Absorption Line Spectrum Definition the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. (a) when a hydrogen atom absorbs. let’s go back to simple absorption and emission spectra. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. you will be able to distinguish between. Absorption Line Spectrum Definition.
From exycgbybx.blob.core.windows.net
Spectra Meaning Spectrum at Lionel Jimenez blog Absorption Line Spectrum Definition You will know how spectral lines are. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of light by a hydrogen atom. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through. Absorption Line Spectrum Definition.
From www.universetoday.com
Absorption Spectroscopy Universe Today Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. Absorption of light by a hydrogen atom. You will know how spectral lines are. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. We. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Absorption Line Spectrum Definition you will be able to distinguish between emission and absorption lines in a spectrum. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. (a) when a hydrogen atom absorbs. We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of light by a. Absorption Line Spectrum Definition.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. let’s go back to simple absorption and emission spectra. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption line will appear in a spectrum if. Absorption Line Spectrum Definition.
From www.astronoo.com
Spectroscopy — Astronoo Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. Absorption of light by a hydrogen atom. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. you will be able to distinguish between emission and absorption lines in a spectrum. let’s go back to simple absorption and emission spectra. an absorption line. Absorption Line Spectrum Definition.
From studylib.net
Continuous, Emission, and Absorption Line Spectra Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. let’s go back to simple absorption and emission spectra. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of light by a hydrogen atom. you will. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Spectrum PowerPoint Presentation, free download Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. (a) when a hydrogen atom absorbs. We can use a star’s absorption spectrum to figure out what elements it is made of. an absorption line will appear in a spectrum if an absorbing material. Absorption Line Spectrum Definition.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption Absorption Line Spectrum Definition let’s go back to simple absorption and emission spectra. You will know how spectral lines are. (a) when a hydrogen atom absorbs. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. Absorption of light by a hydrogen atom. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Chapter 5 Light and Matter Reading Messages from the Cosmos Absorption Line Spectrum Definition We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of light by a hydrogen atom. (a) when a hydrogen atom absorbs. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. let’s go back to simple absorption and emission spectra. the absorption. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Chapter 3 Spectral lines in stars PowerPoint Presentation, free Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. We can use a star’s absorption spectrum to figure out what elements it is made of. Absorption of. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Chapter 3 Spectral lines in stars PowerPoint Presentation, free Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. let’s go back to simple absorption and emission spectra. (a) when a hydrogen atom absorbs. You will know how spectral lines are. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption spectrum is a spectrum obtained when light passes through a medium and. Absorption Line Spectrum Definition.
From www.nagwa.com
Lesson Video Emission and Absorption Spectra Nagwa Absorption Line Spectrum Definition an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. You will know how spectral lines are. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. Absorption of light by a hydrogen atom. We can use a star’s absorption spectrum. Absorption Line Spectrum Definition.
From learninggnibtjb.z21.web.core.windows.net
Atomic Spectra And Its Types Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. You will know how spectral lines are. let’s go back to simple absorption. Absorption Line Spectrum Definition.
From studynonviolent.z13.web.core.windows.net
Absorption Line Spectrum Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. let’s go back to simple absorption and emission spectra. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. You will know how spectral lines are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and. Absorption Line Spectrum Definition.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Absorption Line Spectrum Definition an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. you will be able to distinguish between emission and absorption lines in a spectrum. We can use a star’s absorption. Absorption Line Spectrum Definition.
From www.youtube.com
Emission and Absorption Line Spectrum YouTube Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. We can use a star’s absorption spectrum to. Absorption Line Spectrum Definition.
From sixdayscience.com
Chapter 7 SixDay Science Absorption Line Spectrum Definition the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. an absorption line will appear in a spectrum. Absorption Line Spectrum Definition.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Absorption Line Spectrum Definition We can use a star’s absorption spectrum to figure out what elements it is made of. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. You will know how spectral lines are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and. Absorption Line Spectrum Definition.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. (a) when a hydrogen atom absorbs. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. We can use a star’s absorption spectrum to figure out what elements it is made of. you will be able to distinguish between emission and absorption lines in. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT 07/06/2014 PowerPoint Presentation, free download ID1316566 Absorption Line Spectrum Definition We can use a star’s absorption spectrum to figure out what elements it is made of. let’s go back to simple absorption and emission spectra. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. (a) when a hydrogen atom absorbs. You will know how spectral lines are.. Absorption Line Spectrum Definition.
From pediaa.com
Difference Between Absorption and Emission Spectra Definition Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. let’s go back to simple absorption and emission spectra. you will be able to distinguish between emission and absorption lines in a spectrum. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. We can use a star’s absorption spectrum to figure out what. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Spectral Lines PowerPoint Presentation, free download ID1207109 Absorption Line Spectrum Definition You will know how spectral lines are. you will be able to distinguish between emission and absorption lines in a spectrum. Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. (a) when a hydrogen atom absorbs. the absorption spectrum is a spectrum. Absorption Line Spectrum Definition.
From studynonviolent.z13.web.core.windows.net
What Is The Absorption Line Spectrum Absorption Line Spectrum Definition let’s go back to simple absorption and emission spectra. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. We can use a star’s absorption spectrum to figure out what elements it is made of. you will be able to distinguish between emission and absorption lines in a spectrum. . Absorption Line Spectrum Definition.
From professionalsshery.weebly.com
Color absorbance spectrum professionalsshery Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. You will know how spectral lines are. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. (a) when a hydrogen atom absorbs. . Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Chapter 4 Radiation and Spectra PowerPoint Presentation, free Absorption Line Spectrum Definition an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. you will be able to distinguish between emission and absorption lines in a spectrum. You will know how spectral lines are. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths. Absorption Line Spectrum Definition.
From www.astronomy.ohio-state.edu
Lecture3 Matter and Light Absorption Line Spectrum Definition Absorption of light by a hydrogen atom. (a) when a hydrogen atom absorbs. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. an absorption spectrum is a spectrum obtained. Absorption Line Spectrum Definition.
From fineartamerica.com
Spectrum Of Sun Showing Absorption Lines Photograph by Physics Dept Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. an absorption spectrum is a spectrum obtained when light passes through a medium and certain wavelengths are. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. You will know how spectral lines are. Absorption of light by a hydrogen atom. . Absorption Line Spectrum Definition.
From www.physicsforums.com
Why are absorption spectra continuous? Absorption Line Spectrum Definition let’s go back to simple absorption and emission spectra. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. Absorption of light by a hydrogen atom. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. you will be. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Absorption Line Spectrum Definition We can use a star’s absorption spectrum to figure out what elements it is made of. an absorption line will appear in a spectrum if an absorbing material is placed between a source and the observer. Absorption of light by a hydrogen atom. You will know how spectral lines are. the absorption spectrum is a spectrum of electromagnetic. Absorption Line Spectrum Definition.
From www.slideserve.com
PPT Spectrum PowerPoint Presentation, free download Absorption Line Spectrum Definition (a) when a hydrogen atom absorbs. you will be able to distinguish between emission and absorption lines in a spectrum. the absorption spectrum is a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or. Absorption of light by a hydrogen atom. an absorption spectrum is a spectrum obtained when light passes through a medium. Absorption Line Spectrum Definition.