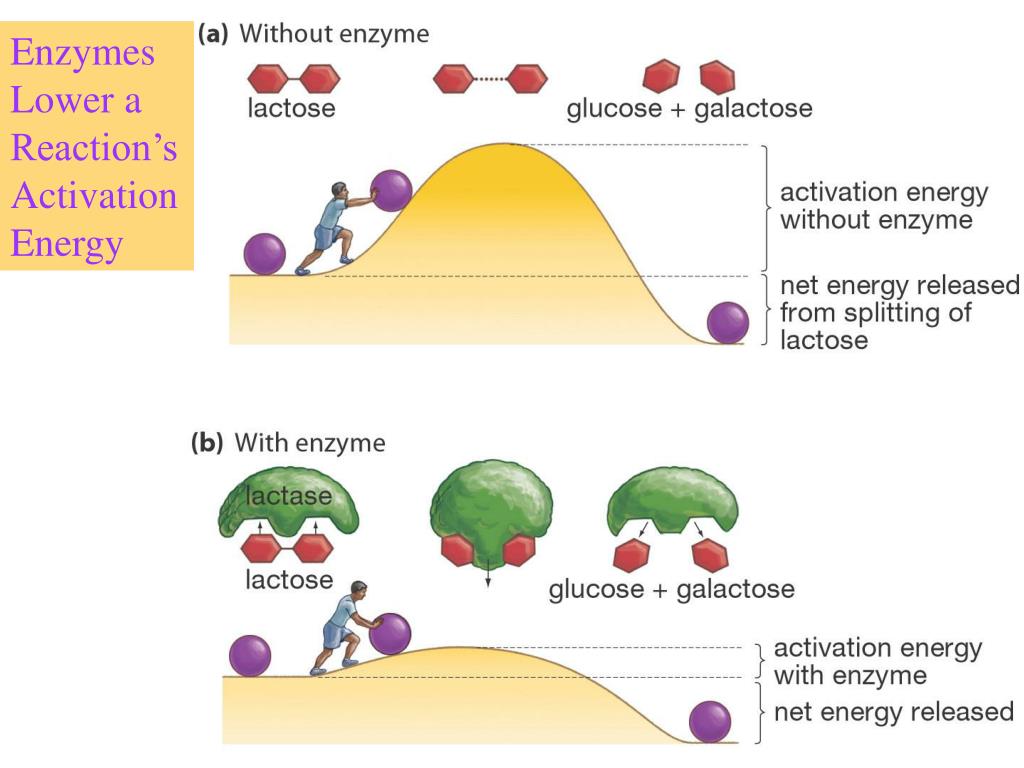
An Enzyme Changes The Activation Energy Of A Chemical Reaction . Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. The increased rate is the same in both. enzymes speed up reactions by lowering the activation energy barrier. — effects of enzymes on activation energy. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. However, if a catalyst is added to the reaction, the activation. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a.
from www.slideserve.com
— effects of enzymes on activation energy. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. enzymes speed up reactions by lowering the activation energy barrier. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. However, if a catalyst is added to the reaction, the activation. The increased rate is the same in both.
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410
An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. The increased rate is the same in both. enzymes speed up reactions by lowering the activation energy barrier. However, if a catalyst is added to the reaction, the activation. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. — effects of enzymes on activation energy. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions.
From www.slideserve.com
PPT Energy, Enzymes, and Biological Reactions PowerPoint Presentation ID669492 An Enzyme Changes The Activation Energy Of A Chemical Reaction an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. However, if a catalyst is added to the reaction, the activation. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. a. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free download ID6848014 An Enzyme Changes The Activation Energy Of A Chemical Reaction — effects of enzymes on activation energy. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. However, if a catalyst is added to the reaction, the activation. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.vrogue.co
The Activation Energy Of Chemical Reactions vrogue.co An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. The increased rate is the same in both. — effects of enzymes on. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 An Enzyme Changes The Activation Energy Of A Chemical Reaction The increased rate is the same in both. enzymes speed up reactions by lowering the activation energy barrier. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. — effects of enzymes on activation energy. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From philschatz.com
Chemical Reactions · Anatomy and Physiology An Enzyme Changes The Activation Energy Of A Chemical Reaction The increased rate is the same in both. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions.. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Chemical Reactions and Enzymes PowerPoint Presentation, free download ID2848082 An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. However, if a catalyst is added to the reaction, the activation. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. — effects of enzymes on activation energy. The increased rate is the same in. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From exowzdqkr.blob.core.windows.net
Enzymes Do Chemical Reaction at Anna Schofield blog An Enzyme Changes The Activation Energy Of A Chemical Reaction — effects of enzymes on activation energy. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. However, if a catalyst is added to the reaction, the activation. enzymes speed up reactions by. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry An Enzyme Changes The Activation Energy Of A Chemical Reaction — effects of enzymes on activation energy. The increased rate is the same in both. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes speed up reactions by lowering the activation energy barrier. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. an enzyme. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. However, if a catalyst is added. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT 7.6 Enzymes (AHL) PowerPoint Presentation, free download ID2742506 An Enzyme Changes The Activation Energy Of A Chemical Reaction Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From schematicrasariopm.z4.web.core.windows.net
Energy Diagram For Chemical Reaction An Enzyme Changes The Activation Energy Of A Chemical Reaction The increased rate is the same in both. — effects of enzymes on activation energy. enzymes speed up reactions by lowering the activation energy barrier. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. However, if a catalyst is added to the reaction, the activation. enzymes (and other catalysts) act by reducing the. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction An Enzyme Changes The Activation Energy Of A Chemical Reaction However, if a catalyst is added to the reaction, the activation. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes speed up reactions by lowering the activation energy barrier. — effects of enzymes on activation energy. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.getbasicidea.com
Introduction of Enzymes An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. enzymes speed up reactions by lowering the activation energy barrier. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. The increased rate is the same in both. — effects of enzymes on activation energy. enzymes in. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. The increased rate is the same in both. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. — effects of enzymes on activation. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. — effects of enzymes on activation energy. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes speed up reactions by lowering the activation energy barrier. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. — effects of enzymes on activation energy. However, if a catalyst. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From telegra.ph
How do enzymes lower activation energy of a reaction Telegraph An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. — effects of enzymes on activation energy. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction.. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID4283724 An Enzyme Changes The Activation Energy Of A Chemical Reaction an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both. enzymes speed up reactions by lowering the activation energy barrier. a substance that helps a chemical. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Chemical Reactions and Enzymes PowerPoint Presentation, free download ID2823212 An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. However, if a catalyst is added to the reaction, the activation. Enzymes are biological catalysts, and therefore not consumed or altered. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From studylib.net
Enzymes Lower Activation Energy An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes speed up reactions by. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From guideelsk5f.z4.web.core.windows.net
Energy Diagram For Chemical Reaction An Enzyme Changes The Activation Energy Of A Chemical Reaction Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. an enzyme is a substance—usually. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From animalia-life.club
Enzyme Activation Energy Graph An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. — effects of enzymes on activation energy. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes.. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From slideplayer.com
Chemical Reactions and Enzymes ppt download An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes speed up reactions by lowering the activation energy barrier. enzymes (and other catalysts) act by reducing. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation energy Ea of the overall An Enzyme Changes The Activation Energy Of A Chemical Reaction Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. The increased rate is the same in both. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes in our bodies are catalysts that speed up reactions by helping to lower. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.britannica.com
Transitionstate theory Definition & Facts Britannica An Enzyme Changes The Activation Energy Of A Chemical Reaction The increased rate is the same in both. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes speed up reactions by lowering the activation energy barrier. — effects of enzymes on activation energy. However, if a. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. — effects of enzymes on activation energy. enzymes speed up reactions by. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Chemical Reactions and Enzymes PowerPoint Presentation, free download ID2823212 An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From savannahkruwhammond.blogspot.com
Enzymes Speed Up Chemical Reactions by Increasing the Activation Energy SavannahkruwHammond An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. — effects. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Activation Energy … PowerPoint Presentation, free download ID4880453 An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. enzymes speed up reactions by lowering the activation energy barrier. enzymes (and. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes speed up reactions by lowering the activation energy barrier. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. The increased rate is the same in both. — effects of enzymes on activation energy. enzymes (and other catalysts) act by reducing the. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.expii.com
What Are Enzymes? — Definition & Overview Expii An Enzyme Changes The Activation Energy Of A Chemical Reaction However, if a catalyst is added to the reaction, the activation. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. enzymes speed up reactions by lowering the activation energy barrier. enzymes in our bodies are catalysts that. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Chapter 6 Basic Concepts of Enzyme Action PowerPoint Presentation ID2739632 An Enzyme Changes The Activation Energy Of A Chemical Reaction Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a. — effects of enzymes on activation energy. enzymes speed up reactions by lowering the activation energy barrier. enzymes (and other catalysts) act. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.chegg.com
Solved How does the molecule labeled A affect enzyme An Enzyme Changes The Activation Energy Of A Chemical Reaction enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. enzymes speed up reactions by lowering the activation energy barrier. a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. The increased rate is the same in both.. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.slideserve.com
PPT Outline of Enzymes PowerPoint Presentation, free download ID364722 An Enzyme Changes The Activation Energy Of A Chemical Reaction a substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. Enzymes are biological catalysts, and therefore not consumed or altered by the reactions. The increased rate is the same in both. — effects of enzymes on activation energy. enzymes speed up reactions by lowering. An Enzyme Changes The Activation Energy Of A Chemical Reaction.
From www.numerade.com
SOLVEDHow does an enzyme change the activation energy for a reaction in a cell? (20.1) An Enzyme Changes The Activation Energy Of A Chemical Reaction an enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. — effects of enzymes on activation energy. enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both. enzymes in our bodies are catalysts that speed up. An Enzyme Changes The Activation Energy Of A Chemical Reaction.