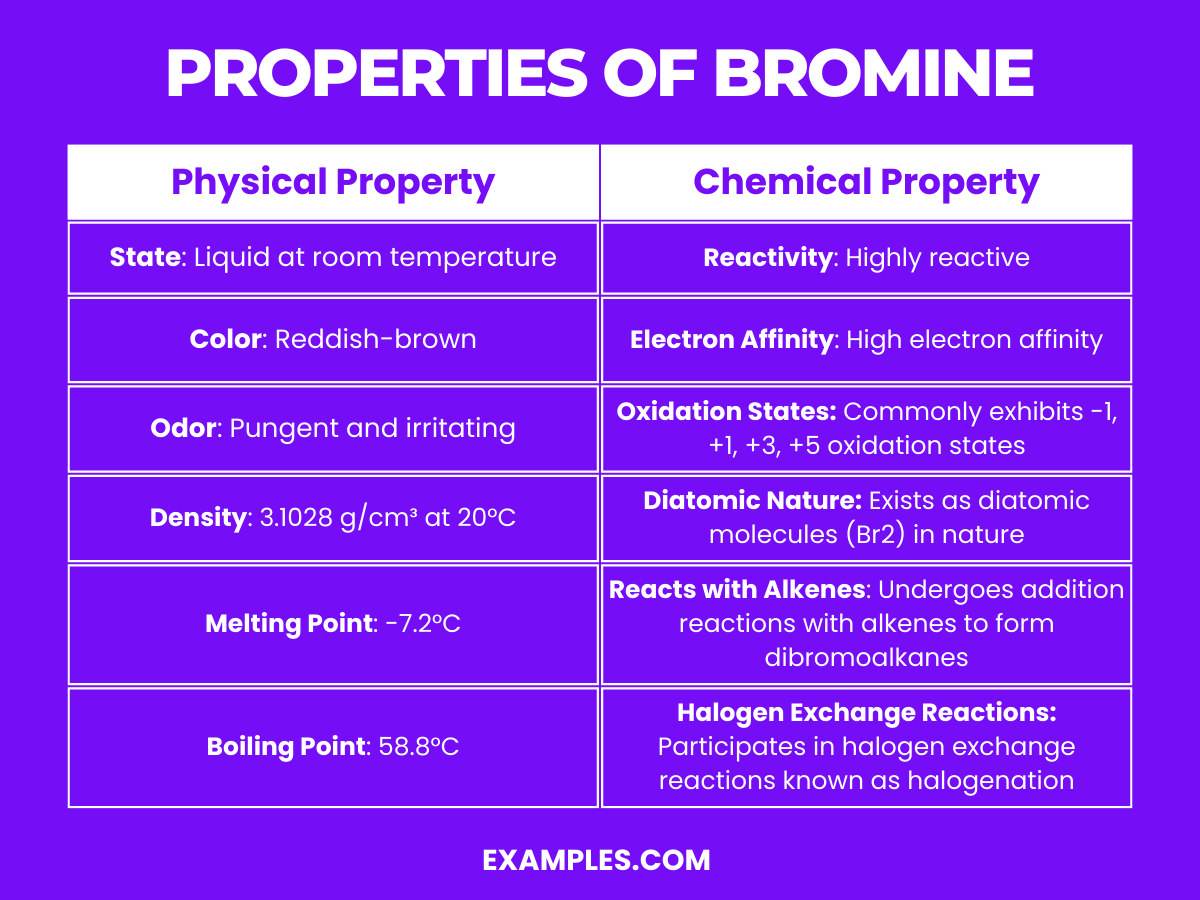
What Properties Do Chlorine And Bromine Have In Common . the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are placed on the right of the periodic table. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. The group 7 elements are also known as the halogens. group 7 halogens properties and uses of the halogens. They are called the halogens because they react with metals to form salts. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts.
from www.examples.com
this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. They are called the halogens because they react with metals to form salts. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. group 7 halogens properties and uses of the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The group 7 elements are placed on the right of the periodic table. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. The group 7 elements are also known as the halogens. the halogens are located on the left of the noble gases on the periodic table.
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity
What Properties Do Chlorine And Bromine Have In Common group 7 halogens properties and uses of the halogens. They are called the halogens because they react with metals to form salts. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are also known as the halogens. group 7 halogens properties and uses of the halogens. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. The group 7 elements are placed on the right of the periodic table. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen.
From www.differencebetween.net
Difference Between Bromine and Chlorine Difference Between What Properties Do Chlorine And Bromine Have In Common They are called the halogens because they react with metals to form salts. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of the noble gases on the periodic table. though chlorine is abundantly present in the earth’s crust and finds many commercial and. What Properties Do Chlorine And Bromine Have In Common.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Between Similar Terms What Properties Do Chlorine And Bromine Have In Common though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. They are called the halogens because they react with metals to form salts. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. The group 7 elements are also known as the. What Properties Do Chlorine And Bromine Have In Common.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison What Properties Do Chlorine And Bromine Have In Common compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. The group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and. What Properties Do Chlorine And Bromine Have In Common.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds, Reactivity What Properties Do Chlorine And Bromine Have In Common compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. They are called the halogens because they react with metals to form salts. group 7 halogens properties and uses of the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The group 7 elements are placed on. What Properties Do Chlorine And Bromine Have In Common.
From www.haikudeck.com
Title by E'lexis Sanders What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. They are called the halogens because they react with metals to form salts. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. . What Properties Do Chlorine And Bromine Have In Common.
From www.slideshare.net
Lecture 6.1 The Periodic Table What Properties Do Chlorine And Bromine Have In Common this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. The group 7. What Properties Do Chlorine And Bromine Have In Common.
From www.buzzle.com
Bromine Vs. Chlorine What Properties Do Chlorine And Bromine Have In Common this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. They are called the halogens because they react with metals to form salts. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. The group 7 elements are placed on the right of. What Properties Do Chlorine And Bromine Have In Common.
From www.youtube.com
Chlorine Gas Physical Properties YouTube What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. The group 7 elements are placed on the right of the periodic table. They are called the halogens because they react with metals to form salts. the halogens are located on the left of the noble gases on the periodic table. compare chlorine and bromine on the basis. What Properties Do Chlorine And Bromine Have In Common.
From www.youtube.com
How To Easily Draw The Mass Spectra Of Chlorine And Bromine Edexcel IAL Chemistry Unit 1 YouTube What Properties Do Chlorine And Bromine Have In Common the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. The group 7 elements are also known as the halogens. the halogens are located on the left of the noble gases on the periodic. What Properties Do Chlorine And Bromine Have In Common.
From www.quora.com
Does Chlorine and Bromine exhibit +4 and +6 oxidation state in oxides and oxoacids? Quora What Properties Do Chlorine And Bromine Have In Common The group 7 elements are placed on the right of the periodic table. group 7 halogens properties and uses of the halogens. the halogens are located on the left of the noble gases on the periodic table. They are called the halogens because they react with metals to form salts. this article contains comparison of key thermal. What Properties Do Chlorine And Bromine Have In Common.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library What Properties Do Chlorine And Bromine Have In Common though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. group 7 halogens properties and uses of the halogens. The group 7 elements are placed on the right of the periodic table. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. . What Properties Do Chlorine And Bromine Have In Common.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table What Properties Do Chlorine And Bromine Have In Common though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. . What Properties Do Chlorine And Bromine Have In Common.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points, Definitions, Examples What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. the halogens are located on the left of the noble gases on the periodic table. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. They are called the halogens because they react with metals to form. What Properties Do Chlorine And Bromine Have In Common.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy What Properties Do Chlorine And Bromine Have In Common the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are placed on the right of the periodic table. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable. What Properties Do Chlorine And Bromine Have In Common.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table What Properties Do Chlorine And Bromine Have In Common The group 7 elements are placed on the right of the periodic table. group 7 halogens properties and uses of the halogens. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are. What Properties Do Chlorine And Bromine Have In Common.
From exoslfhss.blob.core.windows.net
Chlorine Good Definition at Maurice Baez blog What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. They are called the halogens because they react with metals to form salts. group. What Properties Do Chlorine And Bromine Have In Common.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b)... Download Scientific Diagram What Properties Do Chlorine And Bromine Have In Common the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are also known as the halogens. group 7 halogens properties and uses of the halogens. The group 7 elements are placed on the right of. What Properties Do Chlorine And Bromine Have In Common.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. group 7 halogens properties and uses of the halogens. the halogens are located on the left of the noble gases on the periodic table. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. They are called the halogens because they react. What Properties Do Chlorine And Bromine Have In Common.
From blog.intheswim.com
Chlorine vs. Bromine What's the Difference? InTheSwim Pool Blog What Properties Do Chlorine And Bromine Have In Common the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of. What Properties Do Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free download ID9583872 What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of the noble gases on. What Properties Do Chlorine And Bromine Have In Common.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. They are called the halogens because they react with metals to form salts. though chlorine is abundantly present in the earth’s crust and finds many commercial and household. What Properties Do Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID8800875 What Properties Do Chlorine And Bromine Have In Common the halogens are located on the left of the noble gases on the periodic table. group 7 halogens properties and uses of the halogens. The group 7 elements are also known as the halogens. The group 7 elements are placed on the right of the periodic table. They are called the halogens because they react with metals to. What Properties Do Chlorine And Bromine Have In Common.
From exoqkqwbx.blob.core.windows.net
What Properties Does Bromine Have at Glenn Buckley blog What Properties Do Chlorine And Bromine Have In Common the halogens are located on the left of the noble gases on the periodic table. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. group 7 halogens properties and uses of the halogens. compare chlorine and bromine on the basis of their properties, attributes and. What Properties Do Chlorine And Bromine Have In Common.
From pediaa.com
Difference Between Bromine and Chlorine What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. group 7 halogens properties and uses of the halogens. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the. What Properties Do Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 What Properties Do Chlorine And Bromine Have In Common the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. They are called the halogens because they react with metals to form salts. The group 7 elements are also known as the halogens. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. The. What Properties Do Chlorine And Bromine Have In Common.
From feedwater.co.uk
The Feedwater Knowledge base Important water treatment information and news What Properties Do Chlorine And Bromine Have In Common They are called the halogens because they react with metals to form salts. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. group 7 halogens properties and uses of the halogens. The group 7 elements are also known as the halogens. The group 7 elements are. What Properties Do Chlorine And Bromine Have In Common.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity What Properties Do Chlorine And Bromine Have In Common group 7 halogens properties and uses of the halogens. The group 7 elements are also known as the halogens. They are called the halogens because they react with metals to form salts. The group 7 elements are placed on the right of the periodic table. compare chlorine and bromine on the basis of their properties, attributes and periodic. What Properties Do Chlorine And Bromine Have In Common.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b)... Download Scientific Diagram What Properties Do Chlorine And Bromine Have In Common compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The group 7 elements are also known as the halogens. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from. What Properties Do Chlorine And Bromine Have In Common.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. group 7 halogens properties and uses of the halogens. They are called the. What Properties Do Chlorine And Bromine Have In Common.
From www.qcchlorine.com
The Difference Between Chlorine and Bromine in Swimming Pool Disinfection QC Industry What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. group 7 halogens properties and uses of the halogens. the halogens are located on the left of the noble gases on the periodic table. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. though chlorine. What Properties Do Chlorine And Bromine Have In Common.
From utedzz.blogspot.com
Chlorine Bromine Periodic Table Periodic Table Timeline What Properties Do Chlorine And Bromine Have In Common The group 7 elements are placed on the right of the periodic table. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. They are called the halogens because they react with metals to form salts. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from. What Properties Do Chlorine And Bromine Have In Common.
From slideplayer.com
Metals, NonMetals, & Metalloids; Groups / Families & Periods ppt download What Properties Do Chlorine And Bromine Have In Common though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the halogens are located on the left of the noble gases on the periodic table. The group 7 elements are also. What Properties Do Chlorine And Bromine Have In Common.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an. What Properties Do Chlorine And Bromine Have In Common.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Resources What Properties Do Chlorine And Bromine Have In Common the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. though chlorine is abundantly present in the earth’s crust and finds many commercial and household applications, bromine is an equally important. the halogens are located on the left of the noble gases on the periodic table. compare chlorine and bromine on the basis. What Properties Do Chlorine And Bromine Have In Common.
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica What Properties Do Chlorine And Bromine Have In Common The group 7 elements are also known as the halogens. The group 7 elements are placed on the right of the periodic table. the halogens are located on the left of the noble gases on the periodic table. compare chlorine and bromine on the basis of their properties, attributes and periodic table facts. though chlorine is abundantly. What Properties Do Chlorine And Bromine Have In Common.