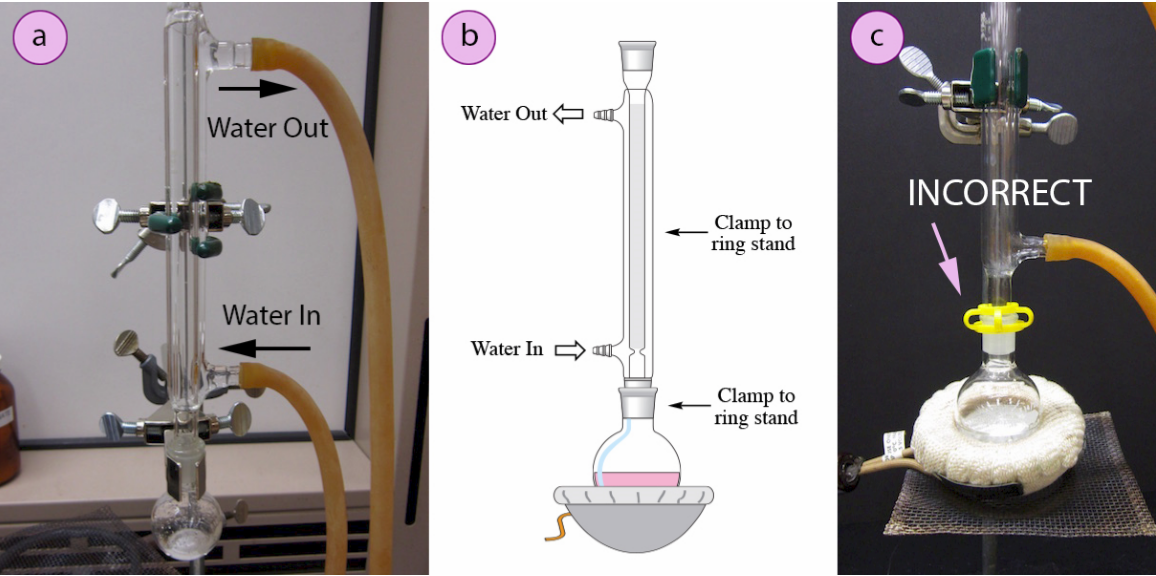
Chemistry Condenser Reflux . The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The solvent which has evaporated from the reaction will condense on the surface of the. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. For reflux boiling, there are 2 very efficient condensers: [1] condensers are routinely used in laboratory. Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The condenser has cold water flowing through it;
from chem.libretexts.org
For reflux boiling, there are 2 very efficient condensers: The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The solvent which has evaporated from the reaction will condense on the surface of the. Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. [1] condensers are routinely used in laboratory. The condenser has cold water flowing through it; Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a.
1.4K Reflux Chemistry LibreTexts
Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The solvent which has evaporated from the reaction will condense on the surface of the. For reflux boiling, there are 2 very efficient condensers: Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The condenser has cold water flowing through it; [1] condensers are routinely used in laboratory. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a.
From imgbin.com
Reflux Condenser Chemistry Chemical Synthesis Roundbottom Flask PNG Chemistry Condenser Reflux The condenser has cold water flowing through it; The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The solvent which. Chemistry Condenser Reflux.
From chemistry.stackexchange.com
experimental chemistry Choosing the right condenser Chemistry Stack Chemistry Condenser Reflux The solvent which has evaporated from the reaction will condense on the surface of the. The condenser has cold water flowing through it; For reflux boiling, there are 2 very efficient condensers: Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a. Chemistry Condenser Reflux.
From catalog.ndsglass.com
Condenser, Reflux, with Joint On NDS Technologies, Inc. Chemistry Condenser Reflux The condenser has cold water flowing through it; In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. For reflux boiling, there are 2. Chemistry Condenser Reflux.
From chemistry.stackexchange.com
safety Safest way to conduct a reaction with bromine under reflux Chemistry Condenser Reflux For reflux boiling, there are 2 very efficient condensers: [1] condensers are routinely used in laboratory. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout. Chemistry Condenser Reflux.
From chem.libretexts.org
1.4K Reflux Chemistry LibreTexts Chemistry Condenser Reflux The condenser has cold water flowing through it; The solvent which has evaporated from the reaction will condense on the surface of the. [1] condensers are routinely used in laboratory. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. For reflux boiling, there are 2. Chemistry Condenser Reflux.
From www.aliexpress.com
200mm Glass Reflux Condenser 24/29 Lab Chemistry Glassware ISO Standard Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. For reflux boiling, there are 2 very efficient condensers: Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve. Chemistry Condenser Reflux.
From www.aliexpress.com
600mm,24/40,Coil Reflux condenser,Laboratory Chemistry Glasswarein Chemistry Condenser Reflux The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. For reflux boiling, there are 2 very efficient condensers: The principle. Chemistry Condenser Reflux.
From fphoto.photoshelter.com
science chemistry equipment reflux condenser lab glass Fundamental Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The condenser has cold water flowing through it; The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. For reflux boiling,. Chemistry Condenser Reflux.
From imgbin.com
Distillation Gastroesophageal Reflux Disease Condenser Acid PNG Chemistry Condenser Reflux For reflux boiling, there are 2 very efficient condensers: The condenser has cold water flowing through it; The solvent which has evaporated from the reaction will condense on the surface of the. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The principle of the reflux operation is. Chemistry Condenser Reflux.
From pubs.acs.org
Lesson Learned from a Fire during Distillation Choose the Appropriate Chemistry Condenser Reflux The solvent which has evaporated from the reaction will condense on the surface of the. For reflux boiling, there are 2 very efficient condensers: Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The condenser has cold water flowing through it; The principle of the. Chemistry Condenser Reflux.
From www.aliexpress.com
200mm,24/29,Coil Reflux glass condenser,Chemistry Laboratory Glassware Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The condenser has cold water flowing through it; Next, the reflux. Chemistry Condenser Reflux.
From chileb.cl
New 200mm 24/29 Glass Straight Liebig Condenser Lab West Condenser Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The condenser has cold water flowing through it; For reflux boiling, there are 2 very efficient condensers: The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while. Chemistry Condenser Reflux.
From ar.inspiredpencil.com
Reflux Apparatus Round Bottom Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. The condenser has cold water flowing through it; In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a.. Chemistry Condenser Reflux.
From www.alamy.com
Сoil condenser, lab chemistry glassware. Glass spiral reflux condenser Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The principle of the reflux operation is. Chemistry Condenser Reflux.
From telgurus.co.uk
What is reflux in chemistry? Why is it used? Chemistry questionnaire. Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. For reflux boiling, there are 2 very efficient condensers: Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are. Chemistry Condenser Reflux.
From www.hotelpresident.ci
Reflux Condenser Diagram hotelpresident.ci Chemistry Condenser Reflux In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. For reflux boiling, there are 2 very efficient condensers: The principle of the reflux. Chemistry Condenser Reflux.
From www.hcs-lab.com
Condenser, Reflux, Large Cooling Capacity, Removable Hose Connections Chemistry Condenser Reflux In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. For reflux boiling, there are 2 very efficient condensers: The condenser has cold water flowing through it; The solvent which has evaporated from the reaction will condense on the surface of the. Reflux involves heating the chemical reaction for. Chemistry Condenser Reflux.
From cartoondealer.com
Lab Apparatus For Reflux, Reflux Is A Technique Involving The Chemistry Condenser Reflux The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The solvent which has evaporated from the reaction will condense on the surface of the. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with. Chemistry Condenser Reflux.
From www.chegg.com
Solved Criticize the following techniques a. A reflux is co Chemistry Condenser Reflux The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The solvent which has evaporated from the reaction will condense on the surface of the. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into. Chemistry Condenser Reflux.
From www.aliexpress.com
300mm,24/40,Glass Spiral Reflux Condenser,New Advance Chemistry Lab Chemistry Condenser Reflux Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The solvent which has evaporated from the reaction will condense on the surface of the. The condenser has cold water flowing through it; [1] condensers are routinely used in laboratory. The. Chemistry Condenser Reflux.
From www.devasthalihotels.com
חברותי עצה גלגל שיניים reflux condenser purpose כותנה מזיקים שעועית Chemistry Condenser Reflux In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The condenser has cold water flowing through it; The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The principle of the reflux operation. Chemistry Condenser Reflux.
From chem.libretexts.org
1.4K Reflux Chemistry LibreTexts Chemistry Condenser Reflux The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. Next, the reflux condenser is attached by means of the ground. Chemistry Condenser Reflux.
From www.researchgate.net
Schematic of reflux setup. Download Scientific Diagram Chemistry Condenser Reflux The solvent which has evaporated from the reaction will condense on the surface of the. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process.. Chemistry Condenser Reflux.
From www.coursehero.com
[Solved] 1.In a reflux set up in an organic chemistry lab what happens Chemistry Condenser Reflux The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. Next, the reflux condenser is attached by means of the ground. Chemistry Condenser Reflux.
From www.hoddereducationmagazines.com
Heating under reflux Hodder Education Magazines Chemistry Condenser Reflux The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Next, the reflux condenser is attached by means of the ground. Chemistry Condenser Reflux.
From roccoscientific.com
Micro Reflux Coil Condenser, 75° Angled Neck Rocco Scientific Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Next, the reflux condenser is attached by. Chemistry Condenser Reflux.
From favpng.com
Reflux Condenser Chemistry Chemical Synthesis Roundbottom Flask, PNG Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. For reflux boiling, there are 2 very efficient condensers: The solvent which has evaporated from the reaction will condense on the surface of the. Reflux involves heating the chemical reaction for a. Chemistry Condenser Reflux.
From blog.soton.ac.uk
Waterless Condensers Turning off the Tap Chemistry SustainAbility Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. In a reflux setup,. Chemistry Condenser Reflux.
From www.thesciencehive.co.uk
Organic Synthesis* — the science sauce Chemistry Condenser Reflux Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. Reflux involves heating the chemical reaction. Chemistry Condenser Reflux.
From www.savemyexams.com
Oxidation of Alcohols SL IB Chemistry Revision Notes 2025 Chemistry Condenser Reflux The solvent which has evaporated from the reaction will condense on the surface of the. For reflux boiling, there are 2 very efficient condensers: [1] condensers are routinely used in laboratory. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. In a reflux setup,. Chemistry Condenser Reflux.
From www.alamy.com
Reflux Condenser High Resolution Stock Photography and Images Alamy Chemistry Condenser Reflux In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The allinh is a good one for general applications, but the dimroth & the double. Chemistry Condenser Reflux.
From themasterchemistry.com
What Is Reflux In Chemistry? A Detailed Guide Chemistry Condenser Reflux Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The solvent which has evaporated from the. Chemistry Condenser Reflux.
From stock.adobe.com
Laboratory setup for reflux reactions. With a Dimroth condenser. Round Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The condenser has cold water flowing through it; The solvent which has evaporated from the reaction will condense on the surface of the. The principle of the reflux operation is to safely provide a controlled amount. Chemistry Condenser Reflux.
From chemistry.stackexchange.com
organic chemistry What is "heating under reflux"? Chemistry Stack Chemistry Condenser Reflux [1] condensers are routinely used in laboratory. Next, the reflux condenser is attached by means of the ground glass joints and another clamp and clamp holder are fixed halfway up the condenser to achieve a better. The condenser has cold water flowing through it; For reflux boiling, there are 2 very efficient condensers: The solvent which has evaporated from the. Chemistry Condenser Reflux.
From www.studocu.com
What is the purpose of a reflux condenser in organic chemistry Answer Chemistry Condenser Reflux Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a. The allinh is a good one for general applications, but the dimroth & the double surface coil condenser can deal with a large amount of. The solvent which has evaporated from the reaction will condense on. Chemistry Condenser Reflux.