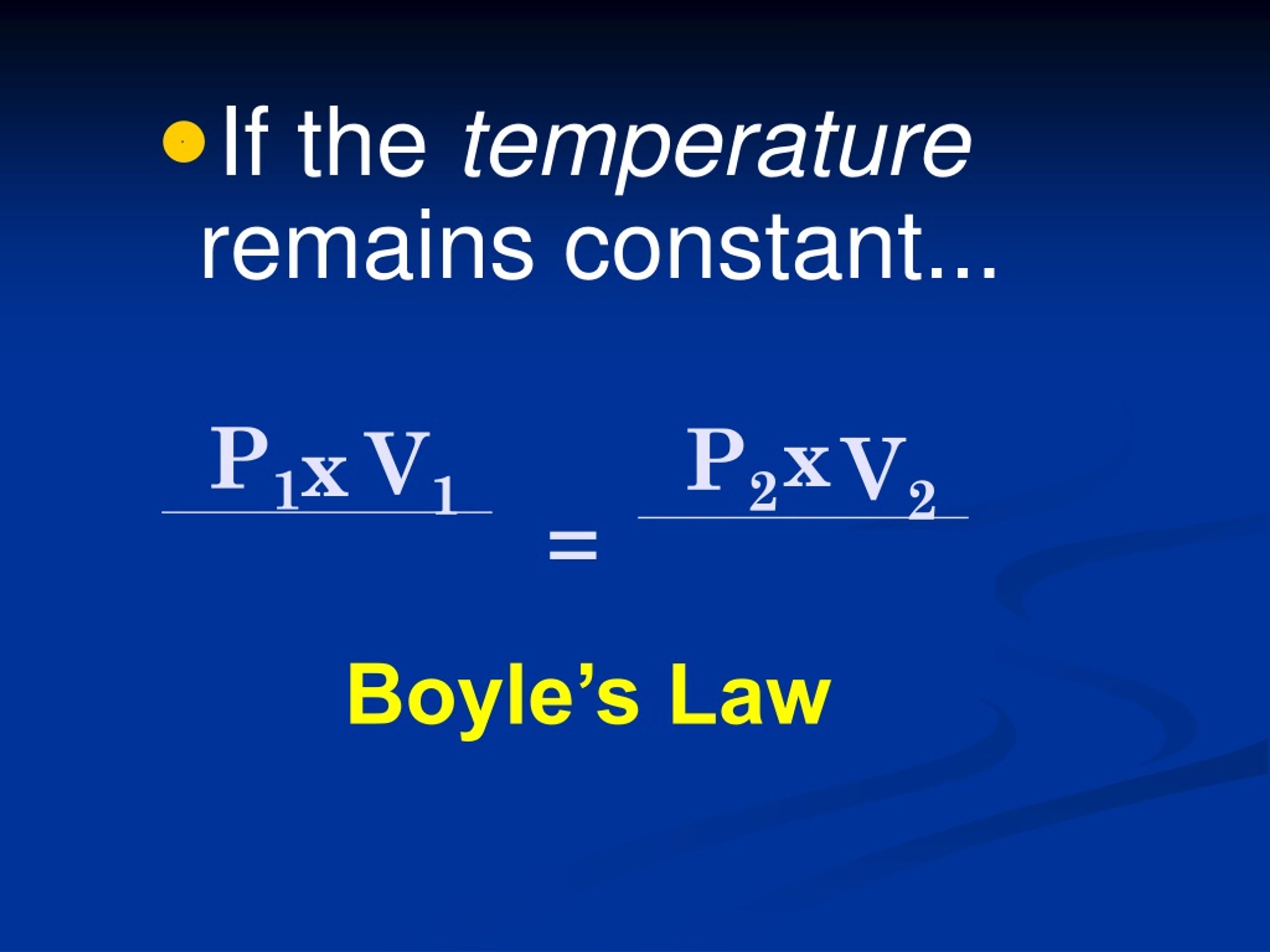
Absolute Pressure Provided The Temperature Remains Constant . Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Charles's law can be succinctly stated as follows: The pressure of a gas at constant volume is a thermometric property because it changes with temperature. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant.
from www.slideserve.com
The pressure of a gas at constant volume is a thermometric property because it changes with temperature. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. Charles's law can be succinctly stated as follows: The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law.
PPT The Nature of Gases PowerPoint Presentation, free download ID
Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant
From www.slideserve.com
PPT The Behavior of Gases PowerPoint Presentation, free download ID Absolute Pressure Provided The Temperature Remains Constant Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume. Absolute Pressure Provided The Temperature Remains Constant.
From www.numerade.com
SOLVED In an isovolumetric process, the temperature remains constant Absolute Pressure Provided The Temperature Remains Constant The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Charles's law can be succinctly stated as follows: The volume of a. Absolute Pressure Provided The Temperature Remains Constant.
From www.chegg.com
Solved Question 19 5 pts Assuming the volume and temperature Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Learn how pressure and. Absolute Pressure Provided The Temperature Remains Constant.
From www.doubtnut.com
Pressure always remains constant Absolute Pressure Provided The Temperature Remains Constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a. Absolute Pressure Provided The Temperature Remains Constant.
From www.chegg.com
Solved As air flows through the duct, its absolute pressure Absolute Pressure Provided The Temperature Remains Constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. Charles's law can be succinctly stated as follows: The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of gas varies inversely with the absolute pressure, provided the. Absolute Pressure Provided The Temperature Remains Constant.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Absolute Pressure Provided The Temperature Remains Constant Charles's law can be succinctly stated as follows: A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of a gas is directly proportional to its absolute temperature. Absolute Pressure Provided The Temperature Remains Constant.
From slideplayer.com
The Behavior of Gases. ppt download Absolute Pressure Provided The Temperature Remains Constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. Charles's law can be succinctly stated as follows: The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of a gas varies inversely to the pressure, provided the. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Constant Pressure Heating of a gas YouTube Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of a gas varies inversely to the pressure, provided. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT HEAT, WORK AND INTERNAL ENERGY PowerPoint Presentation, free Absolute Pressure Provided The Temperature Remains Constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Assume that the temperature remains essentially constant in the upper Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Chapter 12 The Laws of Thermodynamics PowerPoint Presentation Absolute Pressure Provided The Temperature Remains Constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant. Absolute Pressure Provided The Temperature Remains Constant.
From www.coursehero.com
[Solved] Assuming that the temperature remains constant. How can you Absolute Pressure Provided The Temperature Remains Constant The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Absolute Pressure Provided The Temperature Remains Constant Charles's law can be succinctly stated as follows: Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a gas varies inversely to the pressure, provided. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT The Nature of Gases PowerPoint Presentation, free download ID Absolute Pressure Provided The Temperature Remains Constant The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Constant Temperature Process Isothermal Process YouTube Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of. Absolute Pressure Provided The Temperature Remains Constant.
From www.pinterest.com
two beaks filled with liquid and the words boyle's law Absolute Pressure Provided The Temperature Remains Constant Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The pressure of a gas. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Why does the temperature remain constant during a phase transition Absolute Pressure Provided The Temperature Remains Constant The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Charles's law can be succinctly stated as follows: A gas law. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT The Nature of Gases PowerPoint Presentation, free download ID Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Learn how pressure. Absolute Pressure Provided The Temperature Remains Constant.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Charles's law can be succinctly stated as follows: The volume of a. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID6567281 Absolute Pressure Provided The Temperature Remains Constant The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Learn how pressure and temperature are inversely proportional to each other in a. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Theory of Gases PowerPoint Presentation, free download Absolute Pressure Provided The Temperature Remains Constant Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume. Absolute Pressure Provided The Temperature Remains Constant.
From www.numerade.com
SOLVED 7. If the temperature of a gas remains constant but pressure is Absolute Pressure Provided The Temperature Remains Constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Charles's law can be succinctly stated as follows: Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. The pressure of a gas at constant volume is a thermometric property. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Zeroth Law of Thermodynamics PowerPoint Presentation, free Absolute Pressure Provided The Temperature Remains Constant Charles's law can be succinctly stated as follows: The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of a gas varies inversely to the pressure, provided the. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Theory of Gases PowerPoint Presentation, free download Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Charles's law can be succinctly stated as follows: Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant. Absolute Pressure Provided The Temperature Remains Constant.
From www.numerade.com
SOLVED In an isovolumetric process, the temperature remains constant Absolute Pressure Provided The Temperature Remains Constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Charles's law can be succinctly stated as follows: The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Learn how pressure and temperature. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Absolute Pressure Provided The Temperature Remains Constant A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume,. Absolute Pressure Provided The Temperature Remains Constant.
From schematichonorable.z21.web.core.windows.net
Tv Diagram Constant Pressure Absolute Pressure Provided The Temperature Remains Constant The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The pressure of a gas at constant volume is a thermometric property because it changes with temperature. Charles's law can be succinctly stated as follows: Learn how pressure and. Absolute Pressure Provided The Temperature Remains Constant.
From www.doubtnut.com
The pressure of the gas remains constant when its temperature remains Absolute Pressure Provided The Temperature Remains Constant Charles's law can be succinctly stated as follows: The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided. Absolute Pressure Provided The Temperature Remains Constant.
From www.chegg.com
Solved 1. The temperature of an ideal gas remains constant Absolute Pressure Provided The Temperature Remains Constant Charles's law can be succinctly stated as follows: The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume,. Absolute Pressure Provided The Temperature Remains Constant.
From www.grc.nasa.gov
Equation of State Absolute Pressure Provided The Temperature Remains Constant The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. A gas law that states that the absolute pressure of a given quantity of gas varies inversely with its volume provided the temperature remains. Charles's law can be succinctly. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID5724622 Absolute Pressure Provided The Temperature Remains Constant The pressure of a gas at constant volume is a thermometric property because it changes with temperature. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Learn how pressure and temperature are inversely proportional to each other in a fixed amount of gas at constant volume, according to boyle's law. A gas law that. Absolute Pressure Provided The Temperature Remains Constant.
From www.slideserve.com
PPT Unit 5 Gases More Gas Laws Charles’s Law and Boyle’s Law Absolute Pressure Provided The Temperature Remains Constant The volume of a gas varies inversely to the pressure, provided the temperature remains the constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. Learn how pressure and temperature are inversely proportional to each other in a. Absolute Pressure Provided The Temperature Remains Constant.
From www.youtube.com
Constant Temperature Process First Law Of Thermodynamics ) YouTube Absolute Pressure Provided The Temperature Remains Constant The volume of a gas is directly proportional to its absolute temperature when pressure remains constant. The volume of a gas varies inversely to the pressure, provided the temperature remains the constant Charles's law can be succinctly stated as follows: The volume of gas varies inversely with the absolute pressure, provided the temperature remains constant. A gas law that states. Absolute Pressure Provided The Temperature Remains Constant.