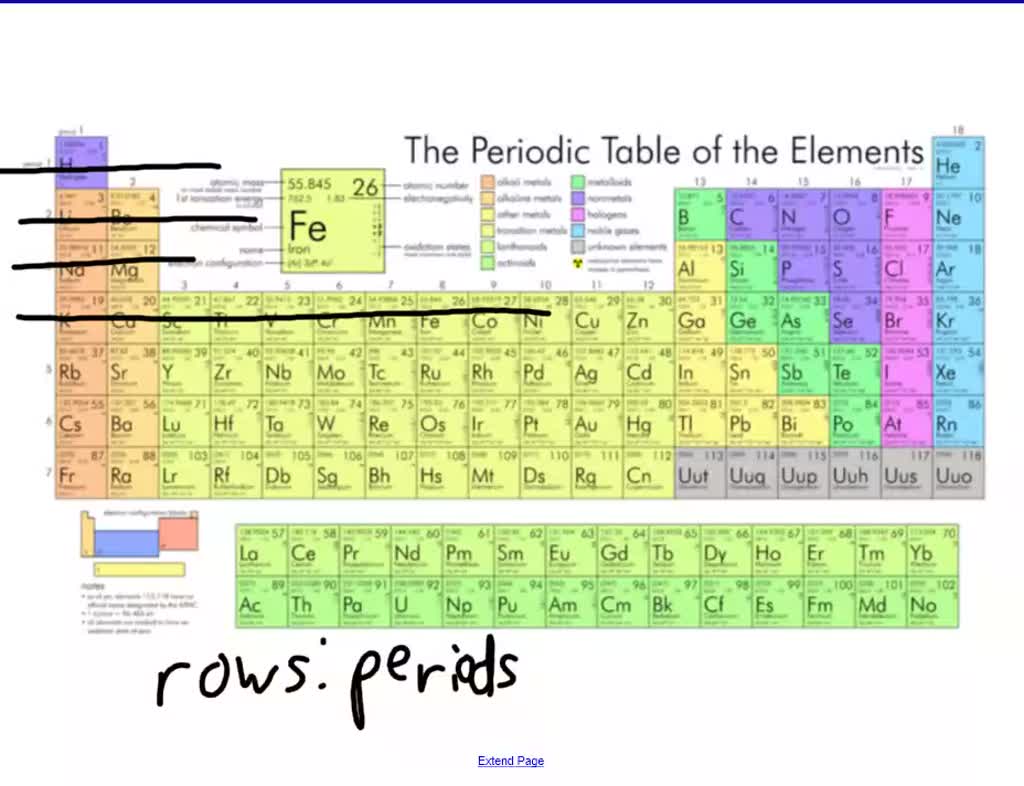
Horizontal Row Of Blocks In The Periodic Table Is . A period is a horizontal row of elements on the periodic table. Both groups and periods reflect the organization of electrons in. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. A horizontal row of blocks in the periodic table is called a(n) a. The period number increases by one for every additional row, up to a maximum of 7. The length of a period depends on how many electrons are needed to occupy the sublevels that. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The periodic table is divided into four block types: For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The horizontal rows of the periodic table are called periods. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Interact with other chemicals by losing, gaining, or sharing the valence.
from awesomehome.co
The periodic table is divided into four block types: Interact with other chemicals by losing, gaining, or sharing the valence. A horizontal row of blocks in the periodic table is called a(n) a. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. The length of a period depends on how many electrons are needed to occupy the sublevels that. The period number increases by one for every additional row, up to a maximum of 7. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The horizontal rows of the periodic table are called periods. A period is a horizontal row of elements on the periodic table.
Periodic Table Rows And Columns Names Awesome Home
Horizontal Row Of Blocks In The Periodic Table Is A period is a horizontal row of elements on the periodic table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. A period is a horizontal row of elements on the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence. Both groups and periods reflect the organization of electrons in. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Each period is given a numerical value, beginning with 1, which is assigned to the top row. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The length of a period depends on how many electrons are needed to occupy the sublevels that. The period number increases by one for every additional row, up to a maximum of 7. The horizontal rows of the periodic table are called periods. A horizontal row of blocks in the periodic table is called a(n) a. The periodic table is divided into four block types:
From period-faqs.com
What Are The Horizontal Rows On The Periodic Table Called Horizontal Row Of Blocks In The Periodic Table Is The horizontal rows of the periodic table are called periods. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. A horizontal row of blocks in the periodic table is called a(n) a.. Horizontal Row Of Blocks In The Periodic Table Is.
From utedzz.blogspot.com
Periodic Table Horizontal Rows Are Called Periodic Table Timeline Horizontal Row Of Blocks In The Periodic Table Is The horizontal rows of the periodic table are called periods. Both groups and periods reflect the organization of electrons in. The periodic table is divided into four block types: Interact with other chemicals by losing, gaining, or sharing the valence. A horizontal row of blocks in the periodic table is called a(n) a. For example, the elements sodium (\(\ce{na}\)) and. Horizontal Row Of Blocks In The Periodic Table Is.
From www.kindpng.com
Periodic Table Labeled Rows And Columns, HD Png Download kindpng Horizontal Row Of Blocks In The Periodic Table Is The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Both groups and periods reflect the organization of electrons in. The periodic table is divided into four block types: Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. A horizontal row of blocks in the periodic table is called a(n) a. A period is a horizontal. Horizontal Row Of Blocks In The Periodic Table Is.
From www.britannica.com
periodic table Definition & Groups Britannica Horizontal Row Of Blocks In The Periodic Table Is The horizontal rows of the periodic table are called periods. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Both groups and periods reflect the organization of electrons in. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a. Horizontal Row Of Blocks In The Periodic Table Is.
From awesomehome.co
Periodic Table Rows And Columns Names Awesome Home Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: Each period is given a numerical value, beginning with 1, which is assigned to the top row. Both groups and periods reflect the organization of electrons in. The period number increases by one for every additional row, up to a maximum of 7. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are. Horizontal Row Of Blocks In The Periodic Table Is.
From utedzz.blogspot.com
Periodic Table Rows Periodic Table Timeline Horizontal Row Of Blocks In The Periodic Table Is A horizontal row of blocks in the periodic table is called a(n) a. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A period is a horizontal row of elements on the periodic table. The horizontal rows of the periodic table are called periods. The period number increases by one for every. Horizontal Row Of Blocks In The Periodic Table Is.
From www.slideserve.com
PPT Blocks in The Periodic Table PowerPoint Presentation, free Horizontal Row Of Blocks In The Periodic Table Is The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Interact with other chemicals by losing, gaining, or sharing the valence. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The period number. Horizontal Row Of Blocks In The Periodic Table Is.
From www.slideserve.com
PPT Periodic Table Geography PowerPoint Presentation, free download Horizontal Row Of Blocks In The Periodic Table Is Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements.. Horizontal Row Of Blocks In The Periodic Table Is.
From slideplayer.com
Periodic Table of Elements how it is arranged how it is used ppt download Horizontal Row Of Blocks In The Periodic Table Is A group is a vertical column down the periodic table, while a period is a horizontal row across the table. A horizontal row of blocks in the periodic table is called a(n) a. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Each period is given a numerical value, beginning with 1, which is assigned. Horizontal Row Of Blocks In The Periodic Table Is.
From chemistrymsq9.blogspot.com
Grade 9 CHAPTER 2 THE PERIODIC TABLE SEMESTER1 Horizontal Row Of Blocks In The Periodic Table Is Interact with other chemicals by losing, gaining, or sharing the valence. A period is a horizontal row of elements on the periodic table. A horizontal row of blocks in the periodic table is called a(n) a. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A group is a vertical column down the periodic table, while a. Horizontal Row Of Blocks In The Periodic Table Is.
From www.storyboardthat.com
Elements and the Periodic Table Vocabulary Storyboard Horizontal Row Of Blocks In The Periodic Table Is The horizontal rows of the periodic table are called periods. The length of a period depends on how many electrons are needed to occupy the sublevels that. Both groups and periods reflect the organization of electrons in. A horizontal row of blocks in the periodic table is called a(n) a. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are. Horizontal Row Of Blocks In The Periodic Table Is.
From www.amnh.org
How to Read the Periodic Table AMNH Horizontal Row Of Blocks In The Periodic Table Is The period number increases by one for every additional row, up to a maximum of 7. Interact with other chemicals by losing, gaining, or sharing the valence. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. A period is a horizontal row of elements on the periodic table. A group is a vertical column down. Horizontal Row Of Blocks In The Periodic Table Is.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The length of a period depends on how many electrons are needed to occupy the sublevels that. A group is a vertical column down the periodic table, while a period is a horizontal row across the table.. Horizontal Row Of Blocks In The Periodic Table Is.
From narodnatribuna.info
Horizontal Row In Periodic Table Period Horizontal Row Of Blocks In The Periodic Table Is For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The period number increases by one for every additional row, up to a maximum of 7. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Both groups and periods reflect the organization of electrons in. The horizontal rows of the periodic table are. Horizontal Row Of Blocks In The Periodic Table Is.
From glossary.periodni.com
Periodic table of the elements Chemistry Dictionary & Glossary Horizontal Row Of Blocks In The Periodic Table Is The length of a period depends on how many electrons are needed to occupy the sublevels that. Each period is given a numerical value, beginning with 1, which is assigned to the top row. Both groups and periods reflect the organization of electrons in. The periodic table is divided into four block types: The horizontal rows of the periodic table. Horizontal Row Of Blocks In The Periodic Table Is.
From www.ck12.org
Families and Periods of the Periodic Table CK12 Foundation Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: The period number increases by one for every additional row, up to a maximum of 7. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A period is a horizontal row of elements on the periodic table. Interact with other chemicals by losing,. Horizontal Row Of Blocks In The Periodic Table Is.
From slideplayer.com
Which of the elements shown has 1 outer electron (D1)? ppt download Horizontal Row Of Blocks In The Periodic Table Is A period is a horizontal row of elements on the periodic table. The horizontal rows of the periodic table are called periods. The periodic table is divided into four block types: The length of a period depends on how many electrons are needed to occupy the sublevels that. Each period is given a numerical value, beginning with 1, which is. Horizontal Row Of Blocks In The Periodic Table Is.
From mavink.com
Periodic Table Horizontal Row Horizontal Row Of Blocks In The Periodic Table Is The length of a period depends on how many electrons are needed to occupy the sublevels that. The horizontal rows of the periodic table are called periods. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The period number increases by one for every additional row, up to a. Horizontal Row Of Blocks In The Periodic Table Is.
From www.welcome-pack.net
A Horizontal Row Of Elements In The Periodic Table Represents Hot Sale Horizontal Row Of Blocks In The Periodic Table Is A horizontal row of blocks in the periodic table is called a(n) a. The horizontal rows of the periodic table are called periods. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Each period is given a numerical. Horizontal Row Of Blocks In The Periodic Table Is.
From elchoroukhost.net
The Rows Of Periodic Table Are Called Elcho Table Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: A horizontal row of blocks in the periodic table is called a(n) a. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The period number increases by one for. Horizontal Row Of Blocks In The Periodic Table Is.
From mavink.com
Periodic Table Row 2 Horizontal Row Of Blocks In The Periodic Table Is The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements.. Horizontal Row Of Blocks In The Periodic Table Is.
From receivinghelpdesk.com
What Are Rows And Columns In A Periodic Table Called Horizontal Row Of Blocks In The Periodic Table Is A horizontal row of blocks in the periodic table is called a(n) a. The periodic table is divided into four block types: Interact with other chemicals by losing, gaining, or sharing the valence. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The horizontal rows of the periodic table are called periods. A period is a horizontal row. Horizontal Row Of Blocks In The Periodic Table Is.
From slidetodoc.com
Periodic Table Electron Configurations The horizontal rows of Horizontal Row Of Blocks In The Periodic Table Is The length of a period depends on how many electrons are needed to occupy the sublevels that. The horizontal rows of the periodic table are called periods. A horizontal row of blocks in the periodic table is called a(n) a. Interact with other chemicals by losing, gaining, or sharing the valence. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both. Horizontal Row Of Blocks In The Periodic Table Is.
From mavink.com
Periodic Table Horizontal Row Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: Each period is given a numerical value, beginning with 1, which is assigned to the top row. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. The horizontal rows of the periodic table are called periods. A horizontal row of. Horizontal Row Of Blocks In The Periodic Table Is.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Horizontal Row Of Blocks In The Periodic Table Is The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Interact with other chemicals by losing, gaining, or sharing the valence. Both groups and periods reflect the organization of electrons in. The horizontal rows of the periodic table are called periods. A horizontal row of blocks in the periodic table is called a(n) a. The period number increases. Horizontal Row Of Blocks In The Periodic Table Is.
From docslib.org
The Periodic Table Horizontal Rows Are Called Periods DocsLib Horizontal Row Of Blocks In The Periodic Table Is The periodic table is divided into four block types: A group is a vertical column down the periodic table, while a period is a horizontal row across the table. A horizontal row of blocks in the periodic table is called a(n) a. Both groups and periods reflect the organization of electrons in. Include carbon, nitrogen, oxygen, sulfur, halogens, and many. Horizontal Row Of Blocks In The Periodic Table Is.
From www.teachoo.com
[Class 10 Term 2] The diagram below shows part of the periodic table Horizontal Row Of Blocks In The Periodic Table Is The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A horizontal row of blocks in the periodic table is called a(n) a. The length of a period depends on how many electrons are needed to occupy the sublevels that. Interact with other chemicals by losing, gaining, or sharing the valence. A group is a vertical column down. Horizontal Row Of Blocks In The Periodic Table Is.
From mavink.com
Periodic Table Horizontal Row Horizontal Row Of Blocks In The Periodic Table Is Each period is given a numerical value, beginning with 1, which is assigned to the top row. A horizontal row of blocks in the periodic table is called a(n) a. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. The horizontal rows of the periodic table are called periods. Both groups and periods reflect the organization of. Horizontal Row Of Blocks In The Periodic Table Is.
From periodictableguide.com
Periods in Periodic table Explained! (With 17 Period Names) Horizontal Row Of Blocks In The Periodic Table Is Each period is given a numerical value, beginning with 1, which is assigned to the top row. The length of a period depends on how many electrons are needed to occupy the sublevels that. Interact with other chemicals by losing, gaining, or sharing the valence. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. The periodic table. Horizontal Row Of Blocks In The Periodic Table Is.
From www.youtube.com
What are horizontal rows in the periodic table known as? QnA Horizontal Row Of Blocks In The Periodic Table Is Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The period number increases by one for every additional row, up to a maximum of 7. A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Interact with other chemicals by losing, gaining, or sharing the valence. A horizontal. Horizontal Row Of Blocks In The Periodic Table Is.
From sciencenotes.org
How To Use a Periodic Table Horizontal Row Of Blocks In The Periodic Table Is A horizontal row of blocks in the periodic table is called a(n) a. The periodic table is divided into four block types: Interact with other chemicals by losing, gaining, or sharing the valence. Each period is given a numerical value, beginning with 1, which is assigned to the top row. The horizontal rows of the periodic table are called periods.. Horizontal Row Of Blocks In The Periodic Table Is.
From cabinet.matttroy.net
A Horizontal Row Of Blocks In The Periodic Table Is Called N Horizontal Row Of Blocks In The Periodic Table Is A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Interact with other chemicals by losing, gaining, or sharing the valence. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. A period is a horizontal row of elements on the periodic table. The elements astatine. Horizontal Row Of Blocks In The Periodic Table Is.
From period-faqs.com
What Are Horizontal Rows On The Periodic Table Called Horizontal Row Of Blocks In The Periodic Table Is A group is a vertical column down the periodic table, while a period is a horizontal row across the table. Each period is given a numerical value, beginning with 1, which is assigned to the top row. A horizontal row of blocks in the periodic table is called a(n) a. Interact with other chemicals by losing, gaining, or sharing the. Horizontal Row Of Blocks In The Periodic Table Is.
From sciencenotes.org
Periodic Table Groups and Periods Horizontal Row Of Blocks In The Periodic Table Is A period is a horizontal row of elements on the periodic table. A horizontal row of blocks in the periodic table is called a(n) a. Each period is given a numerical value, beginning with 1, which is assigned to the top row. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Include carbon, nitrogen, oxygen, sulfur, halogens,. Horizontal Row Of Blocks In The Periodic Table Is.
From utedzz.blogspot.com
Periodic Table With Different Groups Labeled Periodic Table Timeline Horizontal Row Of Blocks In The Periodic Table Is For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The horizontal rows of the periodic table are called periods. A period is a horizontal row of elements on the periodic table. A horizontal row of blocks in the periodic table is called a(n) a. The length of a period depends on how many electrons are. Horizontal Row Of Blocks In The Periodic Table Is.