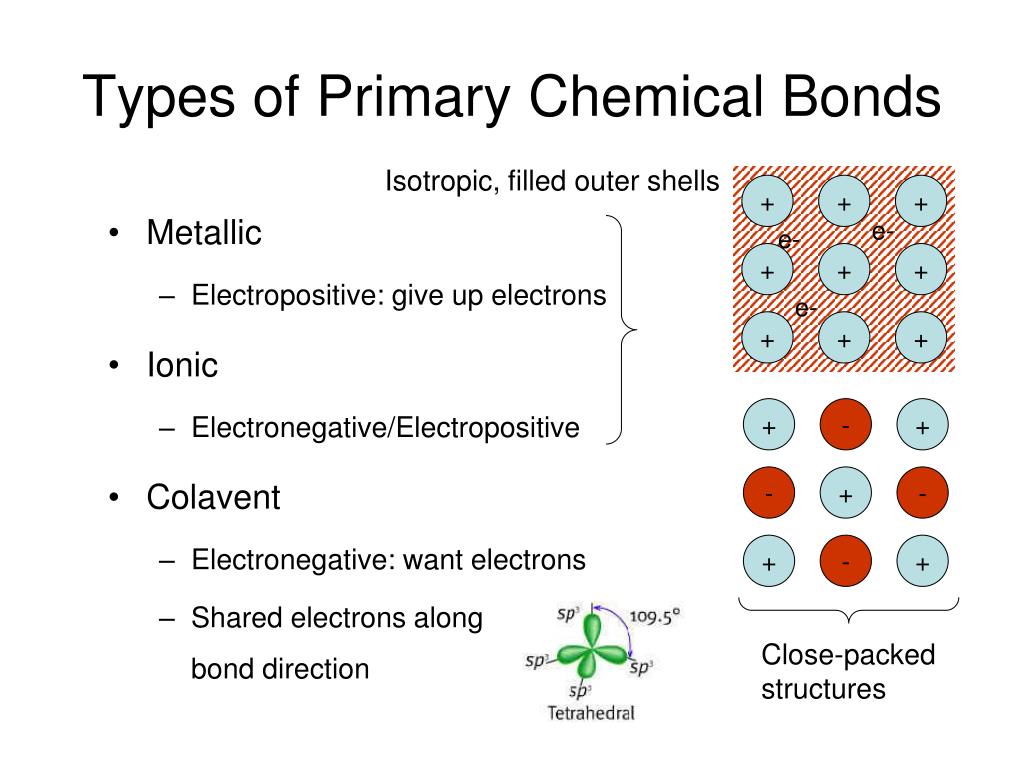
Three Types Of Chemical Bonds In Proteins . Some examples and uses of peptides. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Types and functions of proteins.
from www.slideserve.com
Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Some examples and uses of peptides. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Types and functions of proteins. Peptide bonds are formed by a biochemical reaction that extracts a water molecule.
PPT Types of Primary Chemical Bonds PowerPoint Presentation, free
Three Types Of Chemical Bonds In Proteins Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Some examples and uses of peptides.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Three Types Of Chemical Bonds In Proteins Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Some examples and uses of peptides. Types and. Three Types Of Chemical Bonds In Proteins.
From www.researchgate.net
5 Different levels of protein structure. The amino acids in the Three Types Of Chemical Bonds In Proteins Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Understand a peptide bond and disulfide, their nomenclature, and their characteristics.. Three Types Of Chemical Bonds In Proteins.
From teachmephysiology.com
Protein Structure Amino acids Primary TeachMePhysiology Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Some examples and uses of peptides. Peptide bonds are formed by a biochemical reaction. Three Types Of Chemical Bonds In Proteins.
From www.biologyexams4u.com
What are the 6 Major Chemical Bonds or Interactions In Proteins? Three Types Of Chemical Bonds In Proteins Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Types and functions of proteins. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Understand a. Three Types Of Chemical Bonds In Proteins.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 Three Types Of Chemical Bonds In Proteins Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Important types of bonds involved in protein structure and conformation are peptide. Three Types Of Chemical Bonds In Proteins.
From learningschooljakobi97.z14.web.core.windows.net
Types Of Chemical Bonds Explained Three Types Of Chemical Bonds In Proteins Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Some examples and uses of peptides.. Three Types Of Chemical Bonds In Proteins.
From www.slideserve.com
PPT Mastering Chemistry PowerPoint Presentation, free download ID Three Types Of Chemical Bonds In Proteins Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Some examples and uses of peptides. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Peptide bonds. Three Types Of Chemical Bonds In Proteins.
From chem.libretexts.org
18.4 Proteins Chemistry LibreTexts Three Types Of Chemical Bonds In Proteins Some examples and uses of peptides. Types and functions of proteins. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a. Three Types Of Chemical Bonds In Proteins.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples Three Types Of Chemical Bonds In Proteins Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Some examples and uses of peptides. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Types and functions of proteins. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen.. Three Types Of Chemical Bonds In Proteins.
From mavink.com
Primary Structure Of Protein Diagram Three Types Of Chemical Bonds In Proteins Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Understand a peptide bond and disulfide, their. Three Types Of Chemical Bonds In Proteins.
From www.slideserve.com
PPT Organic and Biological Chemistry PowerPoint Presentation, free Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Types and functions of proteins. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Some examples and uses. Three Types Of Chemical Bonds In Proteins.
From www.britannica.com
crystal Types of bonds Britannica Three Types Of Chemical Bonds In Proteins Some examples and uses of peptides. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains.. Three Types Of Chemical Bonds In Proteins.
From pediaa.com
How are Proteins Constructed from Amino Acids Monomers of Proteins Three Types Of Chemical Bonds In Proteins Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Types and functions of proteins. Polypeptide or. Three Types Of Chemical Bonds In Proteins.
From www.slideserve.com
PPT Types of Primary Chemical Bonds PowerPoint Presentation, free Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Some examples and uses of peptides. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a protein, multiple amino acids are. Three Types Of Chemical Bonds In Proteins.
From www.scienceprofonline.com
What Are Proteins? Amino Acids, Peptide Bonds Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Some examples and uses of peptides. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated.. Three Types Of Chemical Bonds In Proteins.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Proteins Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby. Three Types Of Chemical Bonds In Proteins.
From microbiologynotes.org
Amino acids physical, chemical properties and peptide bond Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Some examples and uses of peptides. Types and functions of proteins. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually. Three Types Of Chemical Bonds In Proteins.
From www.drawittoknowit.com
MCAT Biology & Biochemistry Glossary Protein Structure Class 1 Three Types Of Chemical Bonds In Proteins Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Understand a peptide bond and disulfide, their. Three Types Of Chemical Bonds In Proteins.
From courses.lumenlearning.com
Reading Protein Structure Biology I Three Types Of Chemical Bonds In Proteins Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Some examples and uses of peptides. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Types and functions of proteins.. Three Types Of Chemical Bonds In Proteins.
From www.slideserve.com
PPT Introducing proteins PowerPoint Presentation, free download ID Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Peptide bonds are formed. Three Types Of Chemical Bonds In Proteins.
From cmapspublic.ihmc.us
Proteine Mappa Concettuale Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Some examples and uses of peptides. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Understand. Three Types Of Chemical Bonds In Proteins.
From slcc.pressbooks.pub
7.3 Protein Structure College Biology I Three Types Of Chemical Bonds In Proteins Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Important types of bonds. Three Types Of Chemical Bonds In Proteins.
From cwsimons.com
Structure of Amino Acids and Proteins Three Types Of Chemical Bonds In Proteins Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Types and functions of proteins. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Some examples and. Three Types Of Chemical Bonds In Proteins.
From courses.lumenlearning.com
Proteins Microbiology Three Types Of Chemical Bonds In Proteins Some examples and uses of peptides. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical. Three Types Of Chemical Bonds In Proteins.
From www.bartleby.com
Tertiary Structure of Protein bartleby Three Types Of Chemical Bonds In Proteins Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Some examples and uses of peptides. Understand a peptide bond and disulfide,. Three Types Of Chemical Bonds In Proteins.
From www.thoughtco.com
Four Types of Protein Structure Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Some examples and uses of peptides. Within a protein, multiple amino acids are. Three Types Of Chemical Bonds In Proteins.
From www.biorender.com
Types of Chemical Bonds BioRender Science Templates Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Polypeptide or. Three Types Of Chemical Bonds In Proteins.
From wou.edu
Chapter 2 Protein Structure Chemistry Three Types Of Chemical Bonds In Proteins Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Types and functions of proteins. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Within a protein, multiple amino acids are linked together by peptide bonds, thereby. Three Types Of Chemical Bonds In Proteins.
From courses.lumenlearning.com
Proteins Microbiology Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Some examples and uses of peptides. Types and functions of proteins. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Within a. Three Types Of Chemical Bonds In Proteins.
From sciencenotes.org
Types of Chemical Bonds Three Types Of Chemical Bonds In Proteins Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione). Three Types Of Chemical Bonds In Proteins.
From ditki.com
Biochemistry Glossary Protein Structure an Overview of the Classes Three Types Of Chemical Bonds In Proteins Types and functions of proteins. Some examples and uses of peptides. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and. Three Types Of Chemical Bonds In Proteins.
From courses.lumenlearning.com
Proteins Microbiology Three Types Of Chemical Bonds In Proteins Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Some examples and uses of peptides. Types and functions of proteins. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Polypeptide or protein. Three Types Of Chemical Bonds In Proteins.
From bio.libretexts.org
3.4 The Structure of Proteins Primary Structure Biology LibreTexts Three Types Of Chemical Bonds In Proteins Some examples and uses of peptides. Understand a peptide bond and disulfide, their nomenclature, and their characteristics. Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Types and functions of proteins. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Within a. Three Types Of Chemical Bonds In Proteins.
From www.biologyexams4u.com
What are the 6 Major Chemical Bonds or Interactions In Proteins? Three Types Of Chemical Bonds In Proteins Some examples and uses of peptides. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Enzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated. Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Peptide. Three Types Of Chemical Bonds In Proteins.
From www.youtube.com
Bonds in Protein Structure Biomolecules Biochemistry Three Types Of Chemical Bonds In Proteins Peptide bonds are formed by a biochemical reaction that extracts a water molecule. Important types of bonds involved in protein structure and conformation are peptide bonds, ionic bonds, disulfide bonds, hydrogen. Polypeptide or protein molecules can have anywhere from 3 amino acids (glutathione) to more than 34,000 amino acids (titan) bonded together in chains. Types and functions of proteins. Within. Three Types Of Chemical Bonds In Proteins.