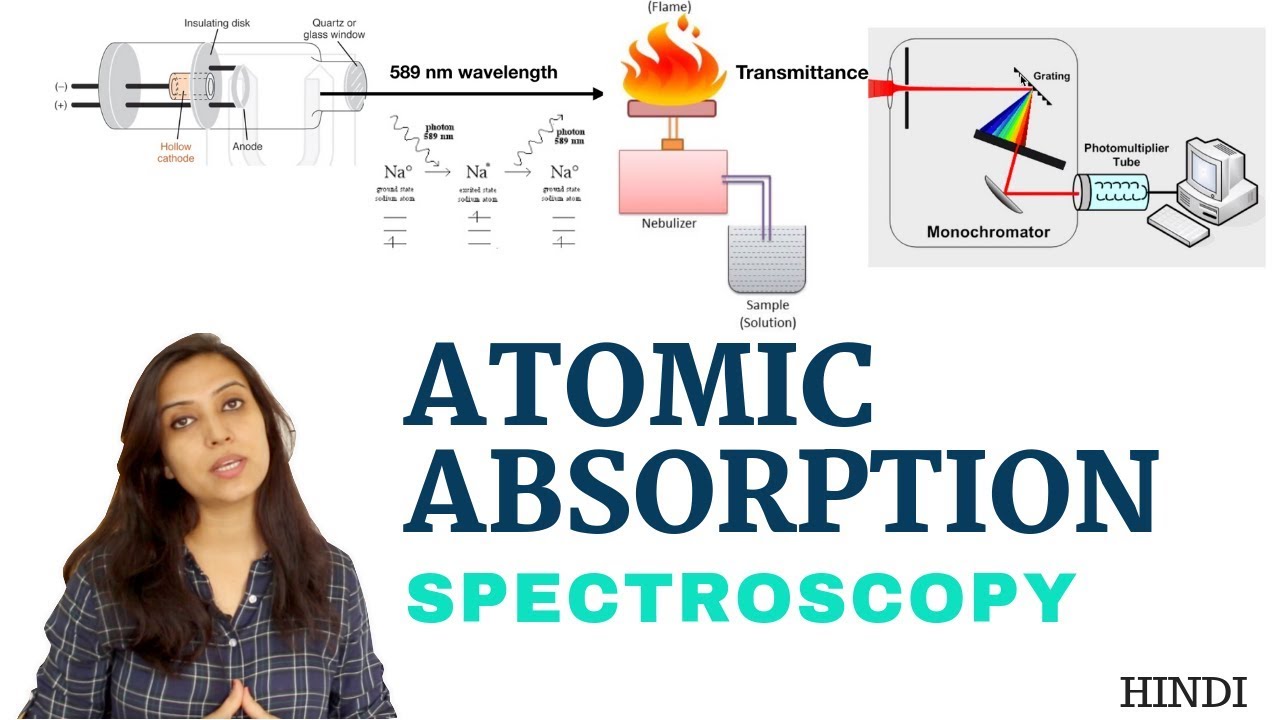
What Does Atomic Absorption Spectroscopy Measure . Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. The light source, the atomization system, the monochromator and the detection system (figure 1). What is atomic absorption spectrometry? Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. A typical atomic absorption spectrometer consists of four main components: An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. It is used to determine the concentration of metals present in a sample to be analyzed.
from www.youtube.com
Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. It is used to determine the concentration of metals present in a sample to be analyzed. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. What is atomic absorption spectrometry? In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. A typical atomic absorption spectrometer consists of four main components:
Atomic Absorption Spectroscopy Introduction & instrumentation YouTube
What Does Atomic Absorption Spectroscopy Measure What is atomic absorption spectrometry? The light source, the atomization system, the monochromator and the detection system (figure 1). What is atomic absorption spectrometry? Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. A typical atomic absorption spectrometer consists of four main components: In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. It is used to determine the concentration of metals present in a sample to be analyzed.
From www.youtube.com
Atomic Absorption Spectroscopy Introduction & instrumentation YouTube What Does Atomic Absorption Spectroscopy Measure What is atomic absorption spectrometry? In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectrometer (AAS) YouTube What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. The light source, the atomization system, the. What Does Atomic Absorption Spectroscopy Measure.
From ibsen.com
Absorption spectroscopy Ibsen Photonics What Does Atomic Absorption Spectroscopy Measure The light source, the atomization system, the monochromator and the detection system (figure 1). It is used to determine the concentration of metals present in a sample to be analyzed. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as. What Does Atomic Absorption Spectroscopy Measure.
From present5.com
Principle of Atomic Absorption Spectrophotometry Mr Charnchai Suracheep What Does Atomic Absorption Spectroscopy Measure A typical atomic absorption spectrometer consists of four main components: It is used to determine the concentration of metals present in a sample to be analyzed. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element. What Does Atomic Absorption Spectroscopy Measure.
From www.studypool.com
SOLUTION Atomic Absorption Spectroscopy Studypool What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. What is atomic absorption spectrometry?. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectroscopy/Atomic Absorption Spectrometry/AAS YouTube What Does Atomic Absorption Spectroscopy Measure What is atomic absorption spectrometry? The light source, the atomization system, the monochromator and the detection system (figure 1). In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. Atomic absorption. What Does Atomic Absorption Spectroscopy Measure.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments What Does Atomic Absorption Spectroscopy Measure An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. It is used to determine the concentration of metals. What Does Atomic Absorption Spectroscopy Measure.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectroscopy (AAS) Explained PART 1 YouTube What Does Atomic Absorption Spectroscopy Measure The light source, the atomization system, the monochromator and the detection system (figure 1). What is atomic absorption spectrometry? An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectrophotometry is. What Does Atomic Absorption Spectroscopy Measure.
From psiberg.com
Atomic Absorption Spectroscopy (AAS) PSIBERG What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. What is atomic absorption spectrometry? A typical atomic absorption spectrometer consists of four main components: In atomic. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT AtomicAbsorption (AA) Spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. A typical atomic absorption spectrometer consists of four main components: In atomic absorption spectroscopy, the wavelength of absorbed light is determined. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomicabsorption spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. It is used to determine the concentration of metals present in a sample to be analyzed. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectroscopy YouTube What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. What is atomic absorption spectrometry? Atomic absorption spectrometry. What Does Atomic Absorption Spectroscopy Measure.
From www.drawellanalytical.com
What is an Atomic Absorption Spectrophotometer & How Does It Work What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. A typical atomic absorption spectrometer consists of four main components: Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. Atomic emission spectroscopy measures the intensity of light emitted by the excited. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectroscopy AAS Forensic Science Instrumentation What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. What is atomic absorption spectrometry? In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Flame Atomic Absorption Spectroscopy Demonstration YouTube What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. The light source, the atomization system, the monochromator and the detection system (figure 1). Atomic absorption spectroscopy (aas) is an absorption. What Does Atomic Absorption Spectroscopy Measure.
From www.slideshare.net
ATOMIC ABSORPTION SPECTROPHOTOMETRY What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. It is used to determine the concentration of metals present in a sample to be analyzed. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. What is atomic absorption spectrometry? Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. A typical atomic absorption spectrometer consists of four main components: The light source, the atomization system, the monochromator and the detection system (figure 1).. What Does Atomic Absorption Spectroscopy Measure.
From users.highland.edu
Atomic Spectra and Models of the Atom What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the.. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy AAS PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. A typical atomic absorption spectrometer consists of four main components: An atomic absorption spectrometer uses these basic. What Does Atomic Absorption Spectroscopy Measure.
From forensicfield.blog
Atomic Absorption Spectroscopy Forensic's blog What Does Atomic Absorption Spectroscopy Measure The light source, the atomization system, the monochromator and the detection system (figure 1). In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. It is used to determine the concentration of metals present in a sample to be analyzed. Atomic absorption spectrometry (aas) detects elements in either. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomicabsorption spectroscopy PowerPoint Presentation, free What Does Atomic Absorption Spectroscopy Measure A typical atomic absorption spectrometer consists of four main components: In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. An atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the. What Does Atomic Absorption Spectroscopy Measure.
From scienceinfo.com
Atomic Absorption Spectroscopy Instrumentation What Does Atomic Absorption Spectroscopy Measure A typical atomic absorption spectrometer consists of four main components: The light source, the atomization system, the monochromator and the detection system (figure 1). In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the. What Does Atomic Absorption Spectroscopy Measure.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation What Does Atomic Absorption Spectroscopy Measure It is used to determine the concentration of metals present in a sample to be analyzed. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. The. What Does Atomic Absorption Spectroscopy Measure.
From www.slideshare.net
Atomic absorption spectrometry (aas) What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. Atomic absorption spectroscopy. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Instrumentation of Atomic absorption spectroscopy YouTube What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. The light source, the atomization system, the monochromator and the detection system (figure 1). In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined. What Does Atomic Absorption Spectroscopy Measure.
From www.youtube.com
Atomic Absorption Spectroscopy & Flame Photometry (Part 1) Principle What Does Atomic Absorption Spectroscopy Measure Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined. What Does Atomic Absorption Spectroscopy Measure.
From www.studypool.com
SOLUTION Atomic Absorption Spectroscopy Studypool What Does Atomic Absorption Spectroscopy Measure In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. A typical atomic absorption spectrometer consists of four main components: Atomic absorption spectrophotometry is a widely used. What Does Atomic Absorption Spectroscopy Measure.
From mothernatureseeker.blogspot.com
Learn about Atomic Absorption Spectroscopy in 5 minutes What Does Atomic Absorption Spectroscopy Measure It is used to determine the concentration of metals present in a sample to be analyzed. Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption. What Does Atomic Absorption Spectroscopy Measure.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrometry (aas) detects elements in either liquid or solid samples through the. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. A typical atomic absorption spectrometer consists of four main components: Atomic absorption spectroscopy (aas) is an absorption spectroscopic method that uses the absorption of. What Does Atomic Absorption Spectroscopy Measure.
From www.analyticalsolns.com.au
What is an Atomic Absorption Spectrophotometer (AAS) Analytical What Does Atomic Absorption Spectroscopy Measure Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in the gas. A typical atomic absorption spectrometer consists of four main components: Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy. In atomic absorption spectroscopy, the wavelength of absorbed light. What Does Atomic Absorption Spectroscopy Measure.
From www.studypool.com
SOLUTION Atomic absorption spectroscopy Studypool What Does Atomic Absorption Spectroscopy Measure It is used to determine the concentration of metals present in a sample to be analyzed. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic absorption spectrophotometry is a widely used analytical technique that involves the measurement of the absorption of electromagnetic radiation by atoms in. What Does Atomic Absorption Spectroscopy Measure.