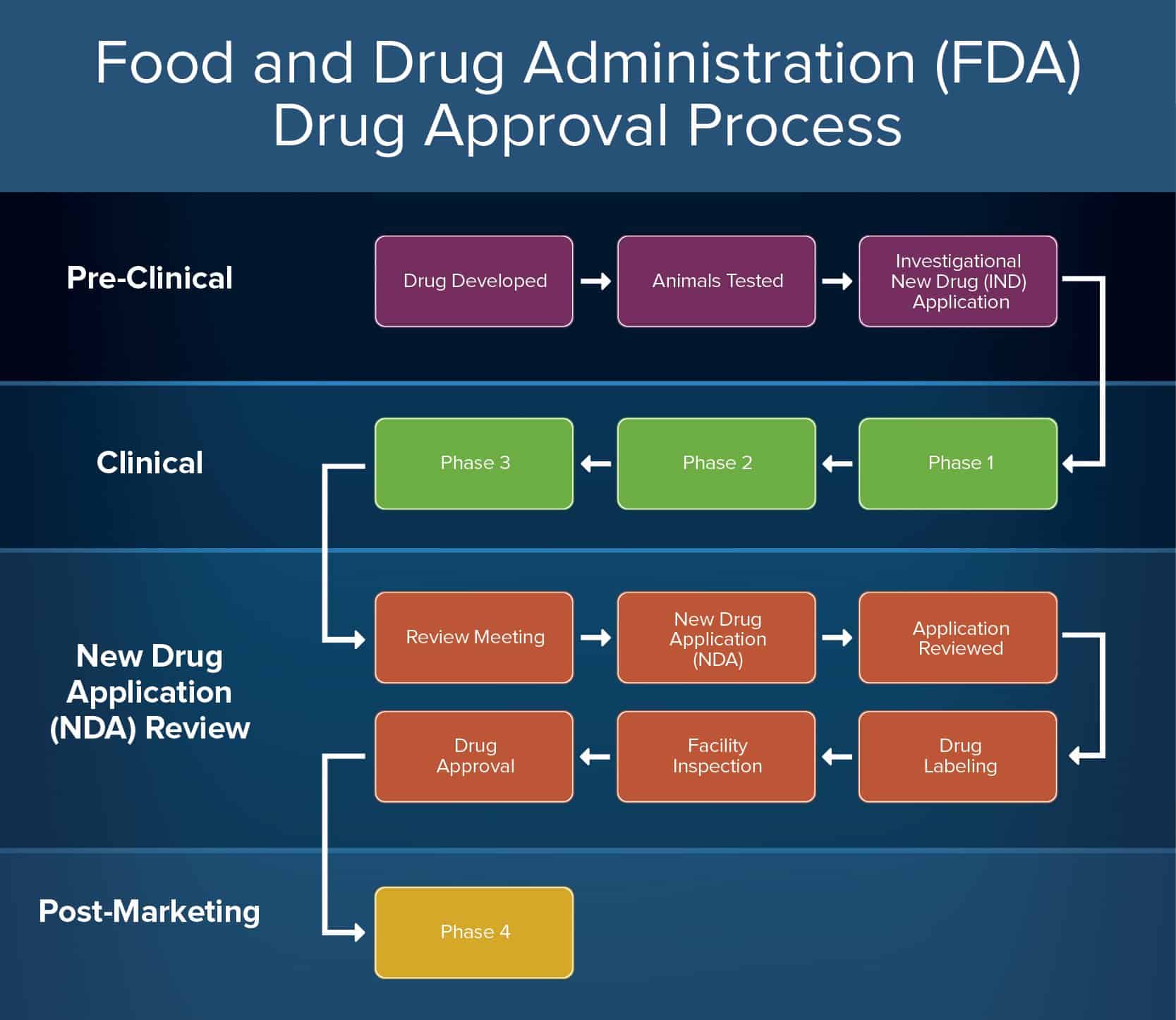
Fda Guidelines For Animal Testing . fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. Food and drug administration (fda) approval, according to. Center for devices and radiological health. This guidance document provides the. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s.
from harryhill.pages.dev
in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. Food and drug administration (fda) approval, according to. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. Center for devices and radiological health. This guidance document provides the. new medicines need not be tested in animals to receive u.s.
Amp 2025 Usfda Guidelines Harry Hill
Fda Guidelines For Animal Testing new medicines need not be tested in animals to receive u.s. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. Center for devices and radiological health. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. This guidance document provides the. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. Food and drug administration (fda) approval, according to.
From colonialchem.com
Animal Testing Colonial Chemical Inc. USMade Chemicals Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. Food and drug administration (fda) approval, according to. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. This guidance document provides the. this document specifies the minimum requirements to be satisfied to ensure and demonstrate. Fda Guidelines For Animal Testing.
From www.drugs.com
FDA Drug Approval Process Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. Center for devices and radiological health. the. Fda Guidelines For Animal Testing.
From www.fda.gov
Animal Rule Information FDA Fda Guidelines For Animal Testing in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. Food and drug administration (fda) approval, according to. Center for devices and radiological health. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. This guidance document provides the. this document specifies the minimum requirements to. Fda Guidelines For Animal Testing.
From theirturn.net
China Ends Animal Testing Requirement on Some Cosmetics Their Turn Fda Guidelines For Animal Testing This guidance document provides the. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. new medicines need not be tested in animals to receive u.s. Center for devices and radiological health. fda developed this. Fda Guidelines For Animal Testing.
From studylib.net
animal testing Fda Guidelines For Animal Testing in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. Food and drug administration (fda) approval, according to. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. This guidance document provides the. Center for devices and radiological health. fda developed this guidance document to assist medical. Fda Guidelines For Animal Testing.
From mungfali.com
Types Of Animal Testing Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. This guidance document provides the. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. this document specifies the minimum. Fda Guidelines For Animal Testing.
From www.fda.gov
What is FDA's budget and what is its impact? Fda Guidelines For Animal Testing this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. This guidance document provides the. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. in issuing its final guidance for. Fda Guidelines For Animal Testing.
From www-patsnap-com.libproxy.mit.edu
FDA Animal Testing 2023 Update PatSnap Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. new medicines need not be tested in animals to receive u.s. This guidance document provides the. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. Center for. Fda Guidelines For Animal Testing.
From www.aplustopper.com
Animal Testing Essay Essay on Animal Testing for Students and Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. This guidance document provides the. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. in issuing its final guidance for animal studies aimed at evaluating medical devices,. Fda Guidelines For Animal Testing.
From www.news-medical.net
Human Health Solutions through Animal Research Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. Center for devices and radiological health. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. new medicines need not be tested in animals to receive u.s. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the. Fda Guidelines For Animal Testing.
From www.slideserve.com
PPT Animal Testing PowerPoint Presentation, free download ID4597865 Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. Center for devices and radiological health. this document specifies the minimum requirements to be. Fda Guidelines For Animal Testing.
From vegnews.com
13 Bipartisan Lawmakers Demand FDA Set Guidelines for AnimalFree Drug Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. new medicines need not be tested in animals to receive u.s. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to. Fda Guidelines For Animal Testing.
From ar.inspiredpencil.com
Animal Testing Statistics Fda Guidelines For Animal Testing Center for devices and radiological health. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. this document specifies the. Fda Guidelines For Animal Testing.
From kids.frontiersin.org
Replacing Animal Testing How and When? · Frontiers for Young Minds Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. the final guidance includes recommendations for various elements of animal studies, including the credentials for. Fda Guidelines For Animal Testing.
From www.eara.eu
FDA Modernization Act 2.0 what, if anything, has changed in US Fda Guidelines For Animal Testing This guidance document provides the. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. Food and drug administration (fda) approval, according. Fda Guidelines For Animal Testing.
From corpbiz.io
Step By Step Process of FDA Certification Fda Guidelines For Animal Testing in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. new medicines need not be tested in animals to receive u.s.. Fda Guidelines For Animal Testing.
From www.understandinganimalresearch.org.uk
Is animal testing ethical? Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. This guidance document provides the. new medicines need not be tested in animals to receive u.s. this document specifies the minimum requirements to be satisfied. Fda Guidelines For Animal Testing.
From worldanimalfoundation.org
Animal Testing Statistics EyeOpening Facts You Must Know! Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. Food and drug administration (fda) approval, according to. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. Center for devices. Fda Guidelines For Animal Testing.
From youragaininfo.blogspot.com
History of animal testing Fda Guidelines For Animal Testing Center for devices and radiological health. This guidance document provides the. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in animals to receive u.s. in issuing its final. Fda Guidelines For Animal Testing.
From plantbasednews.org
Animal Testing Is It Effective, And What Happens To Lab Animals? Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. Center for devices and radiological health. Food and drug administration (fda) approval, according to. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. This guidance document provides the. new medicines need not be tested in animals. Fda Guidelines For Animal Testing.
From www.fda.gov
FDA’s Ongoing Use of Inspectional Tools for Ensuring Access to Safe Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. new medicines need not be tested in animals to receive u.s. Center for devices and radiological health. Food and drug administration (fda) approval, according to. in. Fda Guidelines For Animal Testing.
From blog.biobide.com
Benefits of Animal Testing How Ethical Testing Aids Research Fda Guidelines For Animal Testing Center for devices and radiological health. Food and drug administration (fda) approval, according to. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. the final guidance includes recommendations for various elements of animal studies, including the. Fda Guidelines For Animal Testing.
From xanimalabuse.weebly.com
ANIMAL testing Pros & Cons Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. Center for devices and radiological health. new medicines need not be tested in animals to receive u.s. in issuing its final guidance for animal studies. Fda Guidelines For Animal Testing.
From www.emaze.com
Animal Testing at emaze Presentation Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. Center for devices and radiological health. new medicines need not be tested in animals to receive u.s. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the. Fda Guidelines For Animal Testing.
From www.treehugger.com
Everything You Need to Know About Animal Testing for Cosmetics Fda Guidelines For Animal Testing the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. This guidance document provides the. fda developed this guidance document. Fda Guidelines For Animal Testing.
From vegnews.com
13 Bipartisan Lawmakers Demand FDA Set Guidelines for AnimalFree Drug Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. This guidance document provides the. Food and drug administration (fda) approval, according to. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. Center for devices and radiological health. the final guidance includes recommendations for various elements. Fda Guidelines For Animal Testing.
From www.pcrm.org
FDA Funds Global Collaboration for Innovative Coronavirus Research Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. This guidance document provides the. Center for devices and radiological health. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. new medicines need not be tested in. Fda Guidelines For Animal Testing.
From transcurebioservices.com
The FDA removes animal testing requirement for drug candidates Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. the final guidance includes recommendations for various elements of animal studies,. Fda Guidelines For Animal Testing.
From harryhill.pages.dev
Amp 2025 Usfda Guidelines Harry Hill Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. This guidance document provides the. new medicines need not be tested in animals to receive u.s. this document specifies the minimum requirements to be satisfied. Fda Guidelines For Animal Testing.
From pharmanewsintel.com
The Fundamentals of Animal Testing in Clinical Research Fda Guidelines For Animal Testing Center for devices and radiological health. This guidance document provides the. new medicines need not be tested in animals to receive u.s. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. in issuing its. Fda Guidelines For Animal Testing.
From keplarllp.com
🎉 Drug testing on animals pros and cons. The Pros and Cons of Drug Fda Guidelines For Animal Testing in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. new medicines need not be tested in animals to receive u.s. Food and drug administration (fda) approval, according to. fda developed this guidance document to. Fda Guidelines For Animal Testing.
From www.regdesk.co
FDA on BCI Devices Animal Testing RegDesk Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. This guidance document provides the. in issuing its final guidance. Fda Guidelines For Animal Testing.
From exonsasvl.blob.core.windows.net
Animal Testing Free Videos at Maritza Trevino blog Fda Guidelines For Animal Testing fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. This guidance document provides the. Food and drug administration (fda) approval, according to. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that. Fda Guidelines For Animal Testing.
From blog.biobide.com
Why Animal Testing Is Necessary and How to Reduce It Fda Guidelines For Animal Testing Food and drug administration (fda) approval, according to. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. in issuing its final guidance for animal studies aimed at evaluating medical devices, the u.s. new medicines. Fda Guidelines For Animal Testing.
From www.ncronline.org
Animal welfare groups hail FDA decision to lift animal testing Fda Guidelines For Animal Testing Center for devices and radiological health. this document specifies the minimum requirements to be satisfied to ensure and demonstrate that proper. the final guidance includes recommendations for various elements of animal studies, including the credentials for personnel. fda developed this guidance document to assist medical device sponsors, testing facilities, and other persons. Food and drug administration (fda). Fda Guidelines For Animal Testing.