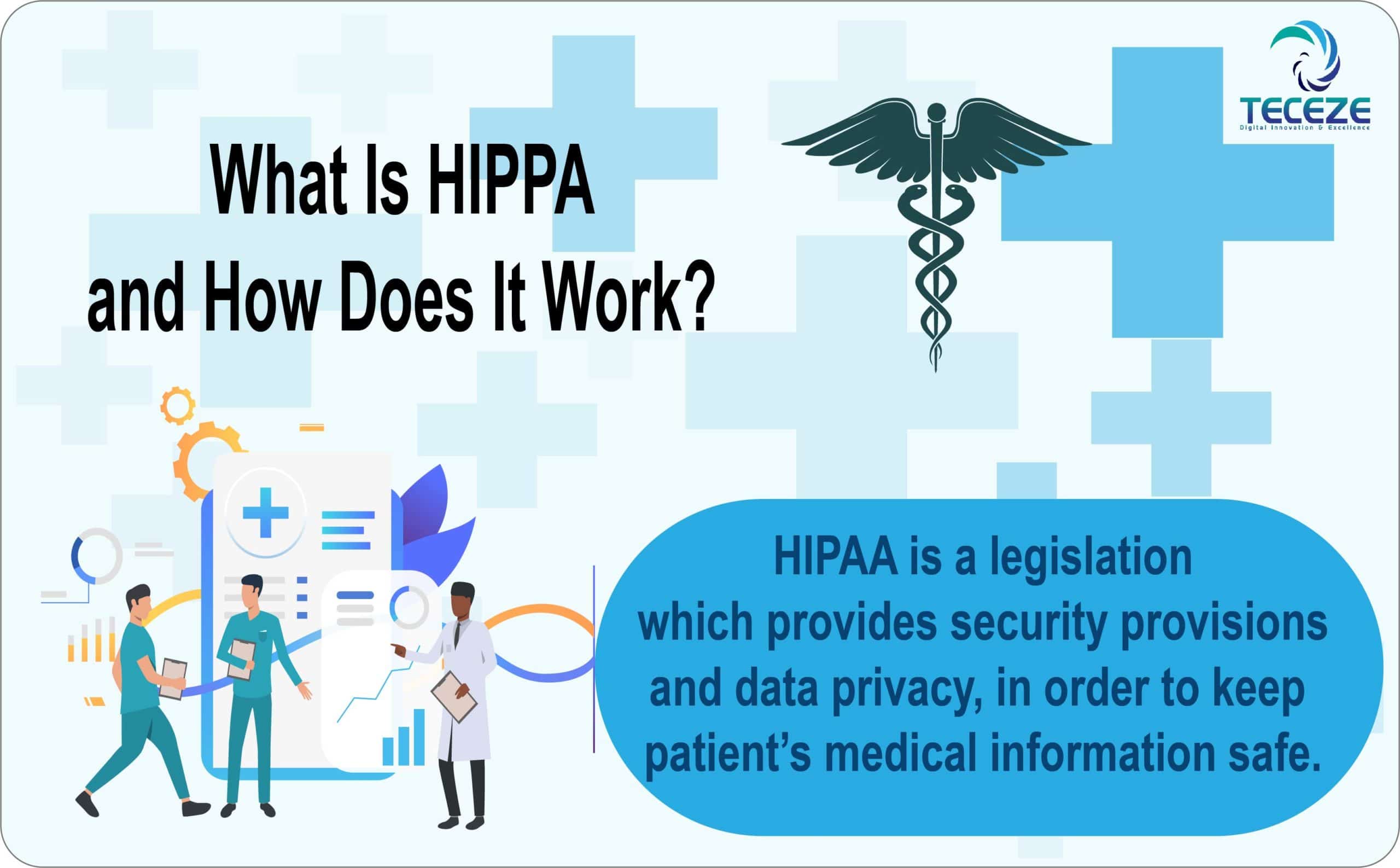
Medical Device Companies And Hipaa . If you are a startup looking to develop. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. there is no defined requirement for medical devices under hipaa. hipaa and gdpr both impact medical device clinical trials. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. Here’s what you need to know about the two regulations governing patient health. An example would be a medical. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. medical device companies commonly fall into the business associates category. in certain situations, a covered health care provider may disclose protected health information to a medical device. the fda regulation of medical devices is conducted via the administration’s center for devices and. Manufacturers need to study the.
from exozalmkr.blob.core.windows.net
An example would be a medical. hipaa and gdpr both impact medical device clinical trials. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Here’s what you need to know about the two regulations governing patient health. If you are a startup looking to develop. in certain situations, a covered health care provider may disclose protected health information to a medical device. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. the fda regulation of medical devices is conducted via the administration’s center for devices and. there is no defined requirement for medical devices under hipaa. medical device companies commonly fall into the business associates category.
Are Medical Devices Covered Under Hipaa at Penny Yawn blog
Medical Device Companies And Hipaa If you are a startup looking to develop. there is no defined requirement for medical devices under hipaa. in certain situations, a covered health care provider may disclose protected health information to a medical device. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. the fda regulation of medical devices is conducted via the administration’s center for devices and. If you are a startup looking to develop. Here’s what you need to know about the two regulations governing patient health. medical device companies commonly fall into the business associates category. An example would be a medical. hipaa and gdpr both impact medical device clinical trials. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. Manufacturers need to study the. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance.
From secureframe.com
The Ultimate HIPAA Compliance Checklist for 2024 Secureframe Medical Device Companies And Hipaa there is no defined requirement for medical devices under hipaa. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. the fda regulation of medical devices is conducted via the administration’s center for devices and. medical devices need to be hipaa compliant, which can pose a serious. Medical Device Companies And Hipaa.
From os-system.com
HIPAA Compliant for Medical App Development Medical Device Companies And Hipaa there is no defined requirement for medical devices under hipaa. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. medical device companies commonly fall into the business associates category. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be.. Medical Device Companies And Hipaa.
From flatirons.com
Top HIPAA Compliant Tools for SaaS Companies Medical Device Companies And Hipaa the fda regulation of medical devices is conducted via the administration’s center for devices and. medical device companies commonly fall into the business associates category. in certain situations, a covered health care provider may disclose protected health information to a medical device. An example would be a medical. hipaa and gdpr both impact medical device clinical. Medical Device Companies And Hipaa.
From exozalmkr.blob.core.windows.net
Are Medical Devices Covered Under Hipaa at Penny Yawn blog Medical Device Companies And Hipaa if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Manufacturers need to study the. the fda regulation of medical devices is conducted via the administration’s center for devices and. An example would be a medical. however, some medical device firms that sell to patients and bill medicare may qualify as. Medical Device Companies And Hipaa.
From www.greenlight.guru
How to Comply with HIPAA and EU GDPR in Medical Device Studies Medical Device Companies And Hipaa If you are a startup looking to develop. medical device companies commonly fall into the business associates category. there is no defined requirement for medical devices under hipaa. An example would be a medical. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. the fda regulation of medical. Medical Device Companies And Hipaa.
From www.pinterest.ca
HIPAA Compliance Guide Hipaa compliance, Hipaa, Healthcare administration Medical Device Companies And Hipaa hipaa and gdpr both impact medical device clinical trials. the fda regulation of medical devices is conducted via the administration’s center for devices and. there is no defined requirement for medical devices under hipaa. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. in certain. Medical Device Companies And Hipaa.
From www.99mgmt.com
[HIPAA Compliance Checklist] Understanding Medical Practice Needs Medical Device Companies And Hipaa An example would be a medical. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. If you are a startup looking to develop. in certain situations, a covered health care provider may disclose protected health information to a medical device. if your medical device transmits, receives, or. Medical Device Companies And Hipaa.
From slchc.edu
What is HIPAA & how does it impact health records? St. Louis College Medical Device Companies And Hipaa If you are a startup looking to develop. there is no defined requirement for medical devices under hipaa. hipaa and gdpr both impact medical device clinical trials. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. in certain situations, a covered health care provider may disclose. Medical Device Companies And Hipaa.
From www.cumulustelecom.com
HIPAA & Medical Devices Medical Device Companies And Hipaa in certain situations, a covered health care provider may disclose protected health information to a medical device. there is no defined requirement for medical devices under hipaa. If you are a startup looking to develop. An example would be a medical. medical device companies commonly fall into the business associates category. however, some medical device firms. Medical Device Companies And Hipaa.
From www.42gears.com
HIPAA Compliance Securing Healthcare Data on Mobile Devices Medical Device Companies And Hipaa if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. in certain situations, a covered health care provider may disclose protected health information to a medical device. there is no defined requirement for medical devices under hipaa. however, some medical device firms that sell to patients and bill medicare may. Medical Device Companies And Hipaa.
From www.practicebuilders.com
How Do you Make a site? Blog Medical Device Companies And Hipaa Manufacturers need to study the. hipaa and gdpr both impact medical device clinical trials. medical device companies commonly fall into the business associates category. there is no defined requirement for medical devices under hipaa. Here’s what you need to know about the two regulations governing patient health. the fda regulation of medical devices is conducted via. Medical Device Companies And Hipaa.
From exozalmkr.blob.core.windows.net
Are Medical Devices Covered Under Hipaa at Penny Yawn blog Medical Device Companies And Hipaa Manufacturers need to study the. Here’s what you need to know about the two regulations governing patient health. the fda regulation of medical devices is conducted via the administration’s center for devices and. there is no defined requirement for medical devices under hipaa. If you are a startup looking to develop. if your medical device transmits, receives,. Medical Device Companies And Hipaa.
From www.infinitepc.net
WHY HIPAA MATTERS ICT Medical Technology Solutions Medical Device Companies And Hipaa Manufacturers need to study the. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. in certain situations, a covered health care provider may disclose protected health information to a medical device. there. Medical Device Companies And Hipaa.
From www.healthcareit.tech
Medical Device Manufacturers HIPAA Training Healthcare IT Medical Device Companies And Hipaa If you are a startup looking to develop. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. Manufacturers need to study the. Here’s what you need to know about the two regulations governing patient health. in certain situations, a covered health care provider may disclose protected health information. Medical Device Companies And Hipaa.
From www.trinsictech.com
Trinsic Modern Technology and HIPAA Compliance Medical Device Companies And Hipaa Here’s what you need to know about the two regulations governing patient health. An example would be a medical. Manufacturers need to study the. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. there is no defined requirement for medical devices under hipaa. however, some medical device firms that. Medical Device Companies And Hipaa.
From www.contractlogix.com
HIPAA Compliant Contracting Software for Healthcare Organizations Medical Device Companies And Hipaa Here’s what you need to know about the two regulations governing patient health. An example would be a medical. hipaa and gdpr both impact medical device clinical trials. If you are a startup looking to develop. Manufacturers need to study the. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities. Medical Device Companies And Hipaa.
From datixinc.com
Maintaining HIPAA Compliance when Collecting PHI in Medical Devices Datix Medical Device Companies And Hipaa there is no defined requirement for medical devices under hipaa. hipaa and gdpr both impact medical device clinical trials. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Manufacturers need to study the. in certain situations, a covered health care provider may disclose protected health information to a medical. Medical Device Companies And Hipaa.
From www.medicaltranscriptionservicecompany.com
Ways to stay HIPAA Compliant when using Mobile Devices Medical Device Companies And Hipaa medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. in certain situations, a covered health care provider may disclose protected health information to a medical device. hipaa and gdpr both impact medical device clinical trials. Manufacturers need to study the. the fda regulation of medical devices is conducted. Medical Device Companies And Hipaa.
From ergos.com
The Essential Role Of HIPAA Compliance In Healthcare ERGOS Medical Device Companies And Hipaa An example would be a medical. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Manufacturers need to study the. the fda regulation of medical devices is conducted via the administration’s center for devices and. hipaa and gdpr both impact medical device clinical trials. medical devices need to be. Medical Device Companies And Hipaa.
From sprinto.com
Who are HIPAA Compliance Officers? Roles and Responsibilities Medical Device Companies And Hipaa An example would be a medical. the fda regulation of medical devices is conducted via the administration’s center for devices and. Manufacturers need to study the. in certain situations, a covered health care provider may disclose protected health information to a medical device. medical devices need to be hipaa compliant, which can pose a serious challenge for. Medical Device Companies And Hipaa.
From www.medicaltranscriptionservicecompany.com
Ways to stay HIPAA Compliant when using Mobile Devices Medical Device Companies And Hipaa If you are a startup looking to develop. there is no defined requirement for medical devices under hipaa. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. the fda regulation of medical devices is conducted via the administration’s center for devices and. medical device companies commonly. Medical Device Companies And Hipaa.
From www.kalleo.net
Risks of Mobile Device Use by Healthcare Organizations What You Must Medical Device Companies And Hipaa An example would be a medical. there is no defined requirement for medical devices under hipaa. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. in certain situations, a covered health care. Medical Device Companies And Hipaa.
From blog.sierralabs.com
Guidance on HIPAA Compliance for Medical Devices Medical Device Companies And Hipaa Here’s what you need to know about the two regulations governing patient health. in certain situations, a covered health care provider may disclose protected health information to a medical device. the fda regulation of medical devices is conducted via the administration’s center for devices and. hipaa and gdpr both impact medical device clinical trials. Manufacturers need to. Medical Device Companies And Hipaa.
From www.truliteled.com
Everything You Need To Know About HIPAA Compliance Trulite Led Medical Device Companies And Hipaa Manufacturers need to study the. in certain situations, a covered health care provider may disclose protected health information to a medical device. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. there is no defined requirement for medical devices under hipaa. Here’s what you need to know. Medical Device Companies And Hipaa.
From www.bluelabellabs.com
How to Make Sure Your App is HIPAA Compliant (Experts Advice) Medical Device Companies And Hipaa Here’s what you need to know about the two regulations governing patient health. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. if your medical device transmits, receives, or records. Medical Device Companies And Hipaa.
From adec-innovations.com
ADEC Innovations Healthcare, Inc. Confirmed as HIPAA Compliant ADEC Medical Device Companies And Hipaa Manufacturers need to study the. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. hipaa and gdpr both impact medical device clinical trials. however, some medical device firms that sell to patients. Medical Device Companies And Hipaa.
From www.arnoldporter.com
HIPAA Refresh What Medical Device and Pharma Companies Need To Know Medical Device Companies And Hipaa there is no defined requirement for medical devices under hipaa. Manufacturers need to study the. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. An example would be a medical. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Here’s. Medical Device Companies And Hipaa.
From exozalmkr.blob.core.windows.net
Are Medical Devices Covered Under Hipaa at Penny Yawn blog Medical Device Companies And Hipaa the fda regulation of medical devices is conducted via the administration’s center for devices and. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. in certain situations, a covered. Medical Device Companies And Hipaa.
From www.neurologyadvisor.com
Protecting Patient Privacy HIPAA Compliance in the Electronic Age Medical Device Companies And Hipaa Here’s what you need to know about the two regulations governing patient health. the fda regulation of medical devices is conducted via the administration’s center for devices and. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. however, some medical device firms that sell to patients and bill medicare. Medical Device Companies And Hipaa.
From www.thehealthcareinvestor.com
As HIPAA Enforcement Efforts Increase, How Should Investors in Medical Device Companies And Hipaa in certain situations, a covered health care provider may disclose protected health information to a medical device. Here’s what you need to know about the two regulations governing patient health. medical device companies commonly fall into the business associates category. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities. Medical Device Companies And Hipaa.
From www.medlineuniversity.com
HIPAA A Guide for Healthcare Workers (1.0 CE for Nurses) Medline Medical Device Companies And Hipaa there is no defined requirement for medical devices under hipaa. the fda regulation of medical devices is conducted via the administration’s center for devices and. however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. hipaa and gdpr both impact medical device clinical trials. If you are. Medical Device Companies And Hipaa.
From www.linkedin.com
Mark DuVal, J.D. FRAPS on LinkedIn Let's Talk About HIPAA Compliance Medical Device Companies And Hipaa An example would be a medical. there is no defined requirement for medical devices under hipaa. the fda regulation of medical devices is conducted via the administration’s center for devices and. If you are a startup looking to develop. if your medical device transmits, receives, or records health information, then it falls under hipaa compliance. Here’s what. Medical Device Companies And Hipaa.
From accentconsulting.com
How IT Can Help Ensure Your Healthcare Practice Is HIPAA Compliant Medical Device Companies And Hipaa however, some medical device firms that sell to patients and bill medicare may qualify as covered entities and be. If you are a startup looking to develop. An example would be a medical. Here’s what you need to know about the two regulations governing patient health. hipaa and gdpr both impact medical device clinical trials. medical devices. Medical Device Companies And Hipaa.
From www.24by7security.com
HIPAA Compliance A practical guide to HIPAA Compliance for a small or Medical Device Companies And Hipaa hipaa and gdpr both impact medical device clinical trials. the fda regulation of medical devices is conducted via the administration’s center for devices and. medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. Here’s what you need to know about the two regulations governing patient health. however, some. Medical Device Companies And Hipaa.
From exozalmkr.blob.core.windows.net
Are Medical Devices Covered Under Hipaa at Penny Yawn blog Medical Device Companies And Hipaa medical devices need to be hipaa compliant, which can pose a serious challenge for manufacturers — especially. there is no defined requirement for medical devices under hipaa. Here’s what you need to know about the two regulations governing patient health. medical device companies commonly fall into the business associates category. if your medical device transmits, receives,. Medical Device Companies And Hipaa.