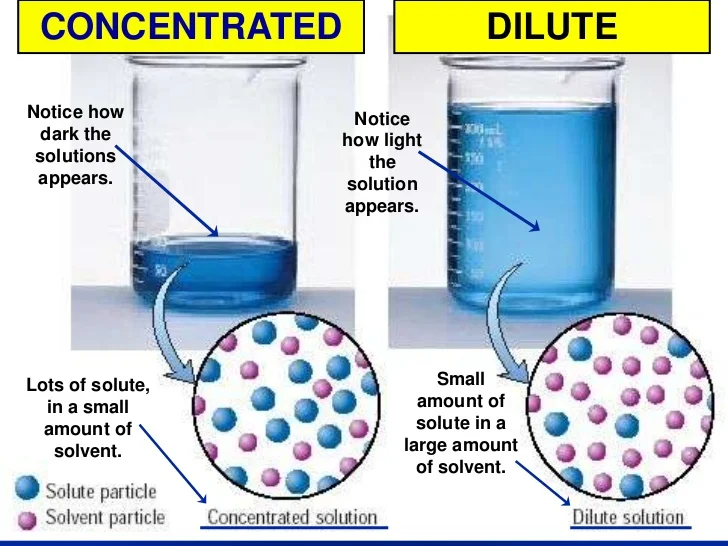
In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution . saturated and unsaturated solutions. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. saturated and unsaturated solutions. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. Suppose that you have a beaker of water to which you add. Miguel added 150 grams of. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Table salt (nacl) readily dissolves in water. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water.
from www.slideshare.net
learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Suppose that you have a beaker of water to which you add. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. saturated and unsaturated solutions. Table salt (nacl) readily dissolves in water. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. Miguel added 150 grams of.
Chapter 16 solutions
In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. saturated and unsaturated solutions. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Suppose that you have a beaker of water to which you add. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. saturated and unsaturated solutions. Table salt (nacl) readily dissolves in water. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. Miguel added 150 grams of.
From www.geeksforgeeks.org
Saturated and Unsaturated Solutions In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Miguel added 150 grams of. describe an example of an unsaturated solution of table salt in 1 liter of 20. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.slideserve.com
PPT Review Next 3 slides PowerPoint Presentation, free download ID In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. a saturated solution is one that contains the maximum amount of solute. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
1. in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Suppose that you have a beaker of water to which you add. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. saturated and unsaturated solutions. learn the difference between saturated and unsaturated solutions, and how to test. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. saturated and unsaturated solutions. saturated and unsaturated solutions. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. describe an example of an. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.youtube.com
Solutions Lesson 1 Solutions and Solubility YouTube In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Suppose that you have a beaker of water to which you add. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. saturated and unsaturated solutions. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. saturated and unsaturated solutions. Table salt \(\left(. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. saturated and unsaturated solutions. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Suppose that you have a beaker of water to which you add. describe an. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Table salt (nacl) readily dissolves in water. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.in
define unsaturated solution Brainly.in In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Miguel added 150 grams of. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. saturated and unsaturated solutions. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. unsaturated solutions are solutions where the amount of dissolved solute is less than the. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
1. In which amount of table salt and water wil form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt (nacl) readily dissolves in water. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. saturated and unsaturated solutions. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. a saturated solution is one that contains the maximum. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Table salt (nacl) readily dissolves in water. Miguel added 150 grams of. Suppose that you have a beaker of water to which you add. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From dxoikfyih.blob.core.windows.net
Liquid Definition Chemistry at Stanley Schreiber blog In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Miguel added 150 grams of. Suppose that you have a beaker of water to which you add. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. saturated and unsaturated solutions. . In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Table salt (nacl) readily dissolves in water. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. saturated and unsaturated solutions. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
1. in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. saturated and unsaturated solutions. Miguel added 150 grams of. Suppose that you have a beaker of water to which you add. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. unsaturated solutions are solutions where the amount of dissolved solute. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From study.com
Saturated Solution Definition & Examples Lesson In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution saturated and unsaturated solutions. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. Suppose that you have a beaker of water to which you add. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. learn the. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Suppose that you have a beaker of water to which you add. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. saturated and unsaturated solutions. saturated and unsaturated solutions. a saturated solution is one that contains the maximum amount of. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.slideserve.com
PPT 06 using a solubility TABLE & SOLUBILITY CURVES PowerPoint In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt (nacl) readily dissolves in water. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Suppose that you have a. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.sciencephoto.com
Water and table salt solution Stock Image C058/7095 Science Photo In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. saturated and unsaturated solutions.. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
Guide Question1. in which amount of table salt and water will form an In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution saturated and unsaturated solutions. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. Table salt (nacl) readily dissolves in water. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. describe an example of an unsaturated solution. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.scribd.com
2 POGIL Saturated and Unsaturated Solutions and Solubility KEY In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. learn how to distinguish between saturated and unsaturated solutions, and how to. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
1. In which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Miguel added 150 grams of. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.neutecgroup.com
Water Activity Standards A Review In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Miguel added. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. saturated and unsaturated solutions. Miguel added 150 grams of. a saturated. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
1. in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Table salt (nacl) readily dissolves in water. Miguel added 150 grams of. Suppose that. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From vinesclassroom.weebly.com
Chemistry Mr. Vines PREPS In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Suppose that you have a beaker of water to which you add. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Miguel added 150 grams of. saturated and unsaturated solutions. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.numerade.com
SOLVED the solubility of table salt (sodium chloride) is 35.7 g/100 g In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Suppose that you have a beaker of water to which you add. saturated and unsaturated solutions. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Miguel added 150 grams of. learn the difference between saturated and unsaturated solutions,. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
Guide Questions1. In which amount of table salt and water will form an In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Table salt (nacl) readily dissolves. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From exomtxqvc.blob.core.windows.net
Table Salt In A Water at Gus Anderson blog In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Suppose that you have a beaker of water to which you add. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. a saturated solution is one that contains the maximum amount. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. saturated and unsaturated solutions. saturated and unsaturated solutions. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Miguel added 150 grams of. describe an example of an unsaturated solution of table salt in 1 liter of 20. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From shaunmwilliams.com
Chapter 11 Presentation In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt (nacl) readily dissolves in water. saturated and unsaturated solutions. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. learn the difference between saturated and unsaturated solutions, and how to test for them using recrystallization. Miguel added 150 grams. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
in which amount of table salt and water will form an unsaturated In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. saturated and unsaturated solutions. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. describe an example of an unsaturated solution of table salt in 1 liter of. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From learningschoolenter1bjf.z4.web.core.windows.net
Solubility And Concentration Worksheet In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. Suppose that you have a beaker of water to which you add. saturated and unsaturated solutions. . In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt (nacl) readily dissolves in water. Suppose that you have a beaker of water to which you add. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. learn the difference between saturated and unsaturated solutions, and how to test for. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From www.slideshare.net
Chapter 16 solutions In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. saturated and unsaturated solutions. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Miguel added 150 grams of. describe an example of an unsaturated solution of table salt in 1 liter of 20 °c water. Suppose that you. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution learn how to distinguish between saturated and unsaturated solutions, and how to achieve solution equilibrium by dissolving. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. a saturated solution is one that contains the maximum amount of solute. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.
From brainly.ph
Guide Questions 1. In which amount of table salt and water will form In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution a saturated solution is one that contains the maximum amount of solute that can dissolve in a given solvent at a. saturated and unsaturated solutions. unsaturated solutions are solutions where the amount of dissolved solute is less than the saturation point of the solvent. saturated and unsaturated solutions. learn how to distinguish between saturated and. In Which Amount Of Table Salt And Water Will Form An Unsaturated Solution.