Does Partial Pressure Increase With Temperature . — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. If you're behind a web filter, please make. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. if you're seeing this message, it means we're having trouble loading external resources on our website. The pressure exerted by each.
from opentextbc.ca
The pressure exerted by each. If you're behind a web filter, please make. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas in a mixture is proportional to its mole fraction.
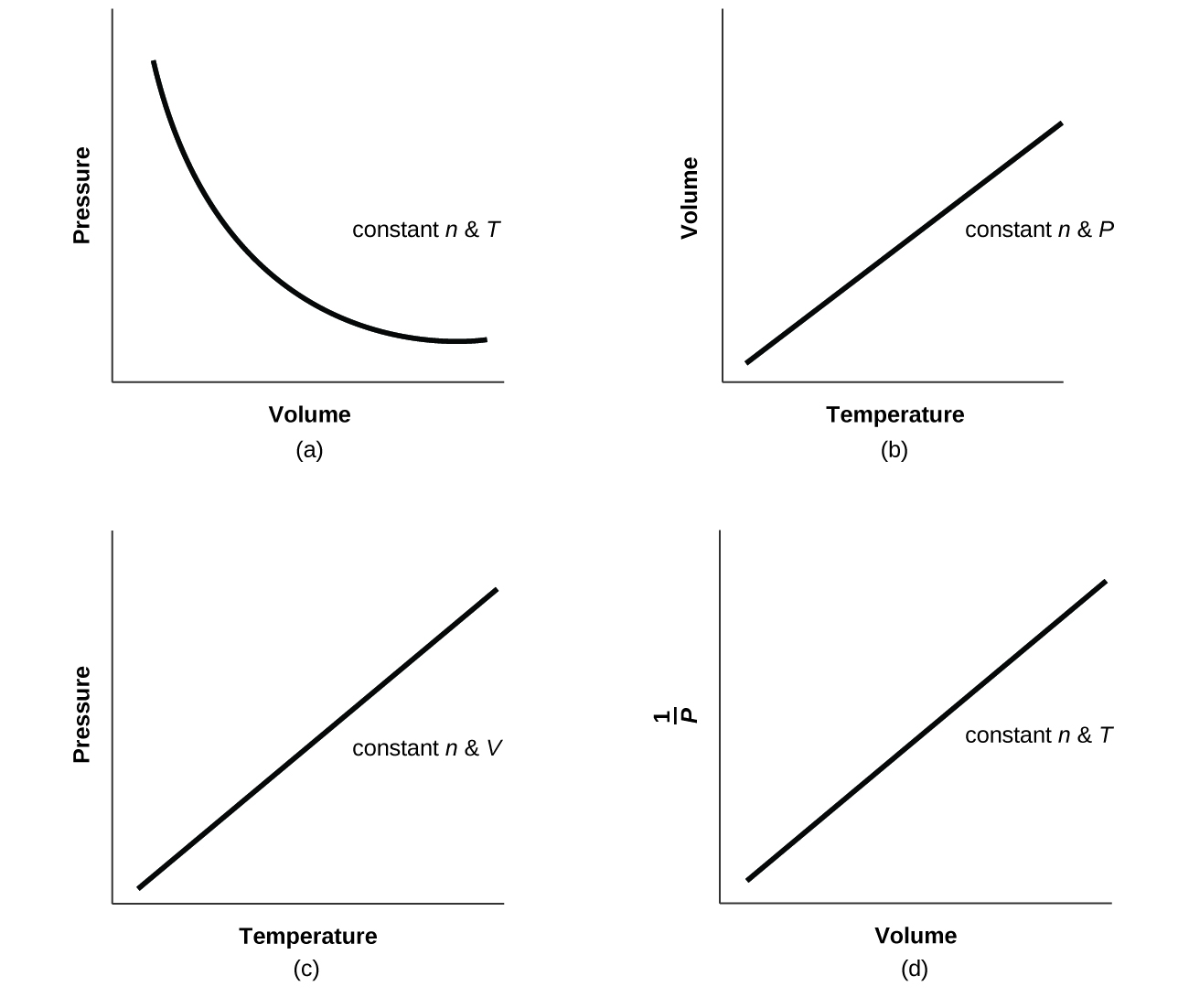
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas
Does Partial Pressure Increase With Temperature — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. If you're behind a web filter, please make. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. The pressure exerted by each. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal.
From dxoithjoo.blob.core.windows.net
Does Pressure Increase With Temperature at Mildred Blose blog Does Partial Pressure Increase With Temperature The pressure exerted by each. If you're behind a web filter, please make. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the law used to find partial pressure assumes the temperature. Does Partial Pressure Increase With Temperature.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket Does Partial Pressure Increase With Temperature — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the. Does Partial Pressure Increase With Temperature.
From www.youtube.com
Law of Partial Pressures YouTube Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. The pressure exerted by each. — the partial pressure of each gas in a mixture is proportional to its mole fraction. if you're seeing this message, it means we're having trouble loading external resources on our website. — the law used to find partial pressure assumes the temperature of. Does Partial Pressure Increase With Temperature.
From byjus.com
Partial Pressure Formula, Dalton's Law, Mixture of Ideal Gas, Examples Does Partial Pressure Increase With Temperature — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the. Does Partial Pressure Increase With Temperature.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Partial Pressure Increase With Temperature — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas in a mixture is proportional to its. Does Partial Pressure Increase With Temperature.
From chemwiki.ucdavis.edu
13.4 Effects of Temperature and Pressure on Solubility Chemwiki Does Partial Pressure Increase With Temperature — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the vapor pressure of a substance depends on both the substance and its. Does Partial Pressure Increase With Temperature.
From www.wikihow.com
How to Calculate Partial Pressure 14 Steps (with Pictures) Does Partial Pressure Increase With Temperature The pressure exerted by each. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. If you're behind a web filter, please make. if you're seeing this message, it means we're having trouble loading. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Does Partial Pressure Increase With Temperature — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. if you're seeing this message, it means we're having trouble loading external resources on our website. — the law used to find partial. Does Partial Pressure Increase With Temperature.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted by each. — the law used to find partial pressure assumes the temperature of. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the partial pressure of each gas in a mixture is proportional to its mole fraction. if you're seeing this message, it means we're having trouble loading external resources on our website. The pressure exerted by. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Gas MixturesPartial Pressure PowerPoint Presentation, free Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. The pressure exerted by each. — the partial pressure of each gas in a mixture is proportional to its mole fraction. —. Does Partial Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure. Does Partial Pressure Increase With Temperature.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Does Partial Pressure Increase With Temperature — the partial pressure of each gas in a mixture is proportional to its mole fraction. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. If you're behind a. Does Partial Pressure Increase With Temperature.
From breathe.ersjournals.com
Relating oxygen partial pressure, saturation and content the Does Partial Pressure Increase With Temperature The pressure exerted by each. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the partial pressure of each gas in a mixture is proportional to its mole fraction. If you're behind a web filter, please make. — the law used to find partial. Does Partial Pressure Increase With Temperature.
From www.researchgate.net
Temperature dependence of the equilibrium oxygen partial pressure for Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure exerted by each. if you're seeing this message, it means we're having trouble loading external resources on our website. — the law used to find partial pressure assumes the temperature of the system is. Does Partial Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Partial Pressure Increase With Temperature — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the partial pressure of each gas in a. Does Partial Pressure Increase With Temperature.
From www.youtube.com
Henry's Law Partial pressure, gas solubility, and mole fraction YouTube Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure exerted by each. if you're seeing this message, it means we're having trouble loading external resources on our website. — the vapor pressure of a substance. Does Partial Pressure Increase With Temperature.
From byjus.com
how partial pressure depends upon temperature explain in detail Does Partial Pressure Increase With Temperature if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make. The pressure exerted by each. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the vapor pressure of a substance. Does Partial Pressure Increase With Temperature.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Does Partial Pressure Increase With Temperature The pressure exerted by each. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the vapor pressure. Does Partial Pressure Increase With Temperature.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Does Partial Pressure Increase With Temperature — the partial pressure of each gas in a mixture is proportional to its mole fraction. if you're seeing this message, it means we're having trouble loading external resources on our website. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following. Does Partial Pressure Increase With Temperature.
From usq.pressbooks.pub
8.5 Transport of Gases Fundamentals of Anatomy and Physiology Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. If you're behind. Does Partial Pressure Increase With Temperature.
From studylib.net
Partial Pressure Does Partial Pressure Increase With Temperature — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure exerted. Does Partial Pressure Increase With Temperature.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure exerted by each. — the vapor pressure of a substance. Does Partial Pressure Increase With Temperature.
From cooljargon.com
Anatomy and Physiology Transport of Gases Does Partial Pressure Increase With Temperature if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the vapor pressure of a. Does Partial Pressure Increase With Temperature.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Does Partial Pressure Increase With Temperature if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted by each. If you're behind a web filter, please make. — the law used to find partial pressure assumes the temperature of. Does Partial Pressure Increase With Temperature.
From www.researchgate.net
Partial Pressure of water vapor vs. temperature, including saturation Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. The pressure exerted by each. If you're behind a web filter, please make. if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of each gas. Does Partial Pressure Increase With Temperature.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Does Partial Pressure Increase With Temperature — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. — the partial pressure of each gas in a mixture is proportional to its mole fraction. If you're behind a web filter, please make. if you're seeing this message, it. Does Partial Pressure Increase With Temperature.
From www.chemistrystudent.com
Partial Pressures (ALevel) ChemistryStudent Does Partial Pressure Increase With Temperature — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas, following the ideal. If you're behind a web filter, please make. if you're seeing this message, it. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT 12.2 DALTON’S LAW OF PARTIAL PRESSURES PowerPoint Presentation Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. If you're behind a web filter, please make. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. if you're seeing this message, it means we're having trouble loading. Does Partial Pressure Increase With Temperature.
From www.youtube.com
Worked example Calculating partial pressures AP Chemistry Khan Does Partial Pressure Increase With Temperature — the partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted by each. if you're seeing this message, it means we're having trouble loading external resources on our website. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID3552130 Does Partial Pressure Increase With Temperature — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted. Does Partial Pressure Increase With Temperature.
From ditki.com
Physiology Glossary Partial Pressures ditki medical & biological Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. The pressure exerted by each. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. — the partial pressure of a gas is the pressure that. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Does Partial Pressure Increase With Temperature If you're behind a web filter, please make. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. if you're seeing this message, it means we're having trouble loading. Does Partial Pressure Increase With Temperature.
From www.slideserve.com
PPT Gases & colligative properties PowerPoint Presentation, free Does Partial Pressure Increase With Temperature if you're seeing this message, it means we're having trouble loading external resources on our website. — the partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself. If you're behind a web filter, please make. — the vapor pressure of a substance depends on both the substance. Does Partial Pressure Increase With Temperature.
From www.youtube.com
Calculate Equilibrium Partial Pressure YouTube Does Partial Pressure Increase With Temperature — the vapor pressure of a substance depends on both the substance and its temperature—an increase in. — the partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted by each. If you're behind a web filter, please make. if you're seeing this message, it means we're having trouble loading. Does Partial Pressure Increase With Temperature.