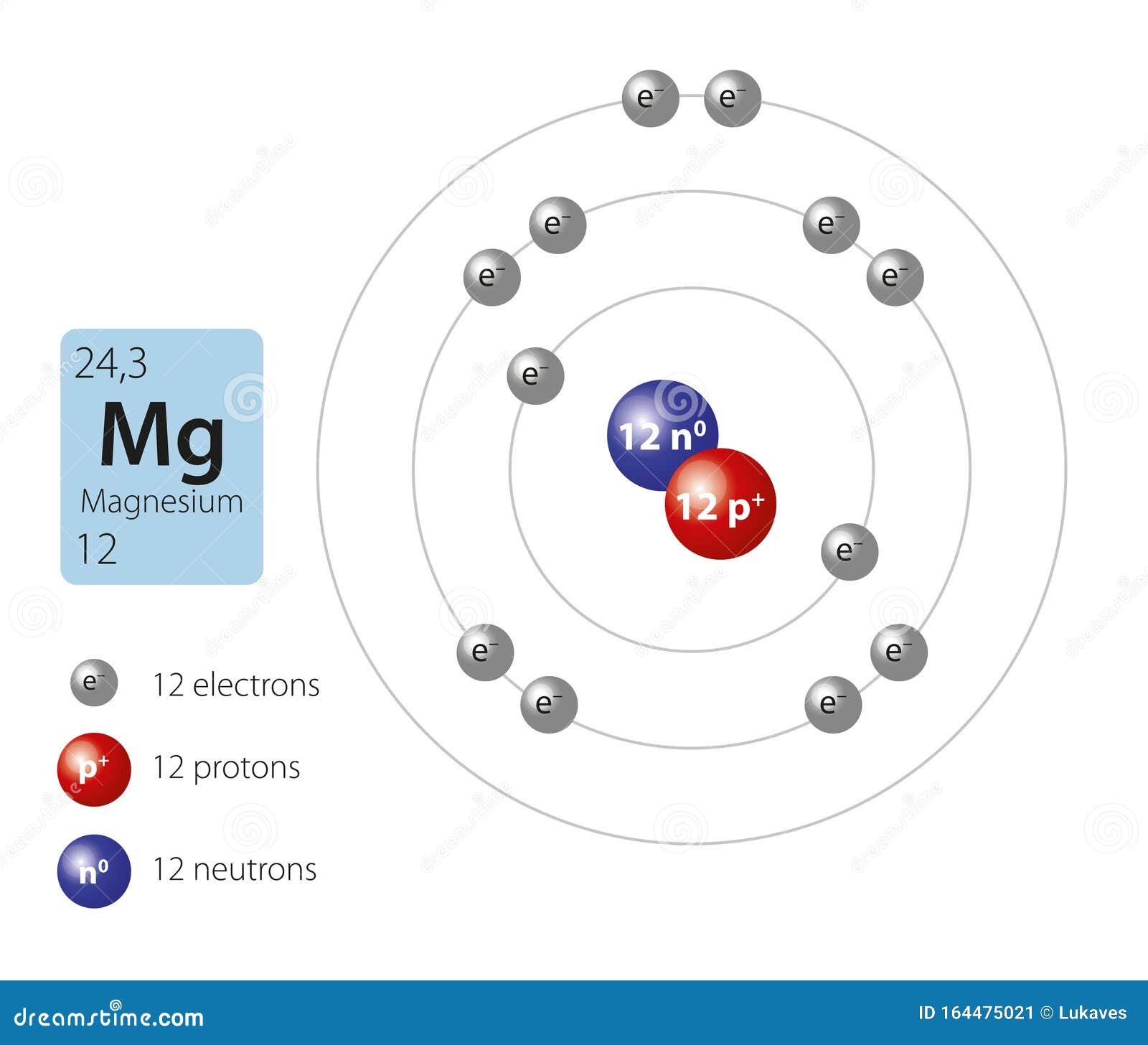
Atomic Mass Of Magnesium Is 24.31 . The average atomic mass of magnesium = 24. The average value is based on their. What is the number of magnesium atoms that equal a mass of 24.31 amu? 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Ohio state university · mechanical. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom.
from www.dreamstime.com
Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. What is the number of magnesium atoms that equal a mass of 24.31 amu? Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. The average value is based on their. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. The average atomic mass of magnesium = 24. Ohio state university · mechanical.
Model of magnesium atom stock vector. Illustration of mass 164475021
Atomic Mass Of Magnesium Is 24.31 We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. What is the number of magnesium atoms that equal a mass of 24.31 amu? Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. The average atomic mass of magnesium = 24. The average value is based on their. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Ohio state university · mechanical.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the. Atomic Mass Of Magnesium Is 24.31.
From loemifrkd.blob.core.windows.net
Magnesium Oxide Atomic Mass at Michael Cruz blog Atomic Mass Of Magnesium Is 24.31 What is the number of magnesium atoms that equal a mass of 24.31 amu? 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. The average value is based on their. Three isotopes, 24 m g, 25 m g and 26 m g of mg can. Atomic Mass Of Magnesium Is 24.31.
From www.chegg.com
Solved Calculate the average atomic mass of magnesium using Atomic Mass Of Magnesium Is 24.31 The average atomic mass of magnesium = 24. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. The average value is based on their. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Ohio state university · mechanical. We know that the atomic masses of. Atomic Mass Of Magnesium Is 24.31.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. Mole % of mg. Atomic Mass Of Magnesium Is 24.31.
From ar.inspiredpencil.com
Magnesium Atomic Number And Mass Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. Ohio state university · mechanical. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. The average value is based on their. The average atomic mass of magnesium = 24. Three isotopes, 24. Atomic Mass Of Magnesium Is 24.31.
From charlee-yersblogbarnes.blogspot.com
Atomic Mass of Magnesium Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. Ohio state university · mechanical. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring. Atomic Mass Of Magnesium Is 24.31.
From brainly.in
average atomic mass of magnesium is 24.31 AMU this magnesium is Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. In a periodic table, the average atomic mass of magnesium is given as 24.312u. We know that the atomic masses of ^24mg and ^25mg. Atomic Mass Of Magnesium Is 24.31.
From www.youtube.com
Calculate the average atomic mass of naturally occurring magnesium Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. The average atomic mass of magnesium = 24. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. Ohio state university · mechanical. Assertion :the average mass of. Atomic Mass Of Magnesium Is 24.31.
From slideplayer.com
Atomic Mass Na The atomic mass of an element ppt download Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. What. Atomic Mass Of Magnesium Is 24.31.
From www.youtube.com
How to Find the Mass of One Atom of Magnesium (Mg) YouTube Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the. Atomic Mass Of Magnesium Is 24.31.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. What is the number of magnesium atoms that equal a mass of 24.31 amu? 1 the atomic mass of magnesium from the periodic table,. Atomic Mass Of Magnesium Is 24.31.
From kunduz.com
[ANSWERED] Atomic mass of magnesium is 24 amu it means that an atom of Atomic Mass Of Magnesium Is 24.31 Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Ohio state university · mechanical. The average value is based on their. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the. Atomic Mass Of Magnesium Is 24.31.
From askfilo.com
Average atomic mass of magnesium is 24.3. This Filo Atomic Mass Of Magnesium Is 24.31 What is the number of magnesium atoms that equal a mass of 24.31 amu? The average atomic mass of magnesium = 24. Ohio state university · mechanical. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. 1 the atomic mass of magnesium from the periodic table,. Atomic Mass Of Magnesium Is 24.31.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Atomic Mass Of Magnesium Is 24.31 Ohio state university · mechanical. The average value is based on their. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. What is the number of. Atomic Mass Of Magnesium Is 24.31.
From www.numerade.com
SOLVEDIn a periodic table the average atomic mass of magnesium is Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. The average value is based on their. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. 1 the atomic mass of magnesium from the periodic table, 24.31. Atomic Mass Of Magnesium Is 24.31.
From www.slideserve.com
PPT Atoms, Ions and Isotopes PowerPoint Presentation ID5401475 Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. Ohio state university · mechanical. The average atomic mass of magnesium = 24. In a periodic table, the. Atomic Mass Of Magnesium Is 24.31.
From www.dreamstime.com
Magnesium Chemical Element with 12 Atomic Number, Atomic Mass and Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. The average atomic mass of magnesium = 24. The average value is based on their. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. In a periodic. Atomic Mass Of Magnesium Is 24.31.
From slideplayer.com
Early models of the atom ppt download Atomic Mass Of Magnesium Is 24.31 The average value is based on their. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. Ohio state university · mechanical. In a periodic table, the average atomic mass of magnesium is given as 24.312u. 1 the atomic mass of magnesium from the periodic table, 24.31. Atomic Mass Of Magnesium Is 24.31.
From circuitlibbottega.z21.web.core.windows.net
Magnesium Diagram Atom Atomic Mass Of Magnesium Is 24.31 What is the number of magnesium atoms that equal a mass of 24.31 amu? Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. The average atomic mass of magnesium = 24. Mole % of mg 24 = 79 mole % of mg 25 and mg 26. Atomic Mass Of Magnesium Is 24.31.
From www.chegg.com
Solved a Verify that the atomic mass of magnesium is 24.31, Atomic Mass Of Magnesium Is 24.31 What is the number of magnesium atoms that equal a mass of 24.31 amu? Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Ohio state university · mechanical. Assertion :the average mass of. Atomic Mass Of Magnesium Is 24.31.
From askfilo.com
Q17. Average atomic mass of magnesium is 24.31 a.m.u. This magnesium is c.. Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. What is the number of magnesium atoms that equal a mass of 24.31 amu? We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. 1 the atomic mass. Atomic Mass Of Magnesium Is 24.31.
From www.coursehero.com
[Solved] Help please and thank you!. The atomic mass of magnesium is 24 Atomic Mass Of Magnesium Is 24.31 The average value is based on their. The average atomic mass of magnesium = 24. In a periodic table, the average atomic mass of magnesium is given as 24.312u. What is the number of magnesium atoms that equal a mass of 24.31 amu? Ohio state university · mechanical. Three isotopes, 24 m g, 25 m g and 26 m g. Atomic Mass Of Magnesium Is 24.31.
From ar.inspiredpencil.com
Magnesium Atom Structure Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg. Atomic Mass Of Magnesium Is 24.31.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Atomic Mass Of Magnesium Is 24.31 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. The average value is based on their. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. In a periodic table, the average. Atomic Mass Of Magnesium Is 24.31.
From www.numerade.com
SOLVEDFor a large collection of magnesium atoms (randomly selected) a Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. What is the number of magnesium atoms that equal a mass of 24.31 amu? Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Three isotopes, 24 m g, 25 m g and 26 m g of mg can. Atomic Mass Of Magnesium Is 24.31.
From www.alamy.com
Magnesium atom, with mass and energy levels. Vector illustration Stock Atomic Mass Of Magnesium Is 24.31 The average atomic mass of magnesium = 24. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one. Atomic Mass Of Magnesium Is 24.31.
From www.doubtnut.com
Average atomic mass of magnesium is 24.31amu. This magnesium is compos Atomic Mass Of Magnesium Is 24.31 Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. In a periodic table, the average atomic mass of magnesium is given as 24.312u.. Atomic Mass Of Magnesium Is 24.31.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Atomic Mass Of Magnesium Is 24.31 Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. What is the number of magnesium atoms that equal a mass of 24.31 amu? The average atomic mass of magnesium = 24. Ohio state university · mechanical. 1 the atomic mass of magnesium from the periodic table,. Atomic Mass Of Magnesium Is 24.31.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Atomic Mass Of Magnesium Is 24.31 Assertion :the average mass of one mg atom is 24.305 amu, which is not equal to the actual mass of one mg atom. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. In. Atomic Mass Of Magnesium Is 24.31.
From slideplayer.com
Chapter 4 Atoms and Elements ppt download Atomic Mass Of Magnesium Is 24.31 Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. What is the number of magnesium atoms that equal a mass of 24.31 amu? The average atomic mass of magnesium = 24. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium,. Atomic Mass Of Magnesium Is 24.31.
From askfilo.com
Average atomic mass of magnesium is 24.31 a.m.u. This magnesium is of 2.. Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. Mole % of mg 24 = 79 mole % of mg 25 and mg 26 = 21. What is the number of magnesium atoms that equal a. Atomic Mass Of Magnesium Is 24.31.
From mungfali.com
Magnesium Atom Diagram Atomic Mass Of Magnesium Is 24.31 In a periodic table, the average atomic mass of magnesium is given as 24.312u. The average value is based on their. Ohio state university · mechanical. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. What is the number of magnesium atoms that equal a. Atomic Mass Of Magnesium Is 24.31.
From www.toppr.com
The average atomic mass of a mixture containing 79 mole percent of ^24 Atomic Mass Of Magnesium Is 24.31 Ohio state university · mechanical. We know that the atomic masses of ^24mg and ^25mg add up to the average atomic mass of magnesium, which is 24.31 amu. The average value is based on their. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Assertion :the average mass of one mg atom is 24.305 amu,. Atomic Mass Of Magnesium Is 24.31.
From www.shutterstock.com
La estructura atómica de magnesio tiene vector de stock (libre de Atomic Mass Of Magnesium Is 24.31 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted average of all of the naturally occurring isotopes of. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. In a periodic table, the average atomic mass of magnesium is given as 24.312u. Mole % of. Atomic Mass Of Magnesium Is 24.31.
From slideplayer.com
Magnesium Magnesium Atomic No. 12 Atomic mass ppt download Atomic Mass Of Magnesium Is 24.31 Ohio state university · mechanical. What is the number of magnesium atoms that equal a mass of 24.31 amu? The average value is based on their. Three isotopes, 24 m g, 25 m g and 26 m g of mg can be found in nature. 1 the atomic mass of magnesium from the periodic table, 24.31 amu, represents the weighted. Atomic Mass Of Magnesium Is 24.31.