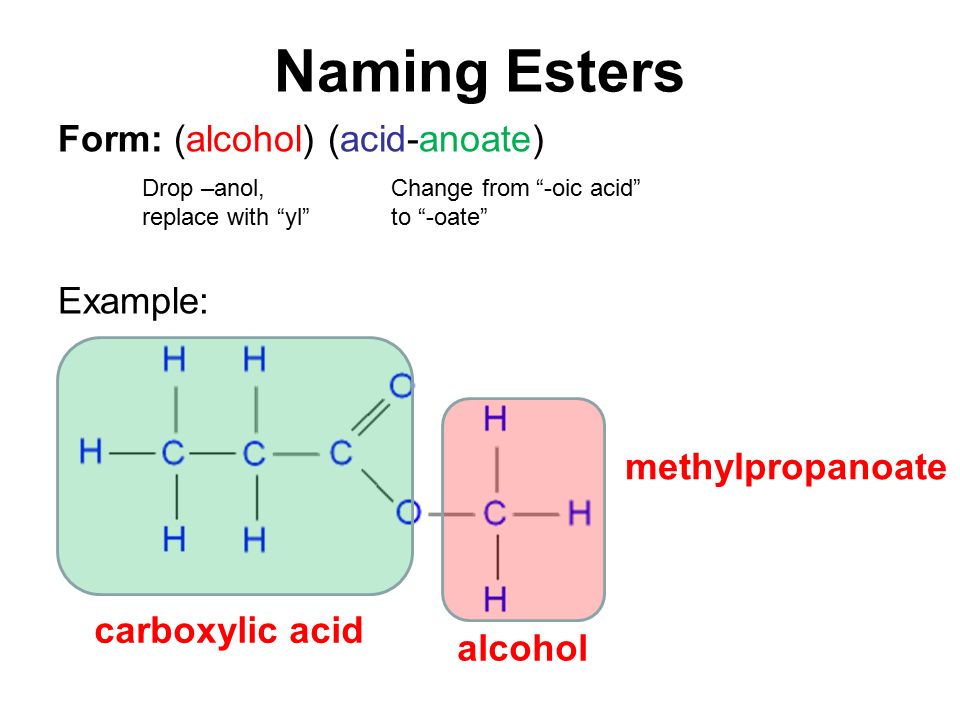
Acetic Acid Ethyl Ester . Chemical reaction of acetic acid and ethanol to produce an ester: Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur through the. Esters occur widely in nature. Esters can also be formed by deprotonating a carboxylic. The odor of vinegar is caused by the presence of acetic acid, a. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Describe the structure and properties of carboxylic acids and esters. Chemistry (openstax), cc by 4.0). Name common carboxylic acids and esters. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. It is widely produced all over the world with an estimation of more than 1 million tons per. Ethyl acetate is the ester of ethanol and acetic acid.
from www.online-sciences.com
Esters can also be formed by deprotonating a carboxylic. Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Describe the structure and properties of carboxylic acids and esters. Chemical reaction of acetic acid and ethanol to produce an ester: Esters occur widely in nature. The odor of vinegar is caused by the presence of acetic acid, a. This can occur through the. Name common carboxylic acids and esters. It is widely produced all over the world with an estimation of more than 1 million tons per.
Physical and chemical properties of Esters, Esterification reaction and
Acetic Acid Ethyl Ester Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Describe the structure and properties of carboxylic acids and esters. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Chemistry (openstax), cc by 4.0). The odor of vinegar is caused by the presence of acetic acid, a. This can occur through the. Esters can also be formed by deprotonating a carboxylic. Ethyl acetate is the ester of ethanol and acetic acid. It is widely produced all over the world with an estimation of more than 1 million tons per. Name common carboxylic acids and esters. Chemical reaction of acetic acid and ethanol to produce an ester: Esters occur widely in nature.
From www.youtube.com
Give a chemical equation for the reaction between ethyl alcohol and Acetic Acid Ethyl Ester This can occur through the. Chemical reaction of acetic acid and ethanol to produce an ester: Esters occur widely in nature. The odor of vinegar is caused by the presence of acetic acid, a. Name common carboxylic acids and esters. Chemistry (openstax), cc by 4.0). It is widely produced all over the world with an estimation of more than 1. Acetic Acid Ethyl Ester.
From sielc.com
Benzeneacetic acid, .alpha.(2,4dinitrophenyl)2,4dinitro, ethyl Acetic Acid Ethyl Ester Esters can also be formed by deprotonating a carboxylic. Ethyl acetate is the ester of ethanol and acetic acid. Chemistry (openstax), cc by 4.0). This can occur through the. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. It is widely produced all over the world with an estimation of more than 1 million tons per. Chemical. Acetic Acid Ethyl Ester.
From en.wikipedia.org
Ethyl acetate Wikipedia Acetic Acid Ethyl Ester Esters occur widely in nature. Chemistry (openstax), cc by 4.0). The odor of vinegar is caused by the presence of acetic acid, a. Esters can also be formed by deprotonating a carboxylic. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. This can occur through the.. Acetic Acid Ethyl Ester.
From www.fishersci.se
Ethyl acetate, 99, Thermo Scientific Chemicals Fisher Scientific Acetic Acid Ethyl Ester Chemical reaction of acetic acid and ethanol to produce an ester: Esters occur widely in nature. Describe the structure and properties of carboxylic acids and esters. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Ethyl acetate is the ester of ethanol and acetic acid. It. Acetic Acid Ethyl Ester.
From www.alamy.com
Ethyl acetate, ethyl ethanoate, C4H8O2 molecule. It is acetate ester Acetic Acid Ethyl Ester Name common carboxylic acids and esters. Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Chemistry (openstax), cc by 4.0). Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple. Acetic Acid Ethyl Ester.
From www.sielc.com
Acetic acid, nitro, ethyl ester SIELC Acetic Acid Ethyl Ester This can occur through the. Chemistry (openstax), cc by 4.0). Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Chemical reaction of acetic acid and ethanol to produce an ester: Describe the structure and properties of carboxylic acids and esters. Esters can also be formed by deprotonating. Acetic Acid Ethyl Ester.
From www.dreamstime.com
Ethyl Acetate, Ethyl Ethanoate, C4H8O2 Molecule. it is Acetate Ester Acetic Acid Ethyl Ester Describe the structure and properties of carboxylic acids and esters. It is widely produced all over the world with an estimation of more than 1 million tons per. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur through the. Acid ahydrides and carboxylic acids can also react with alcohols. Acetic Acid Ethyl Ester.
From www.coursehero.com
[Solved] Acetic acid and ethanol react to form an ester product as Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. Chemistry (openstax), cc by 4.0). Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur through the. Chemical reaction of acetic acid and ethanol to produce an ester: Esters can also be formed by deprotonating a carboxylic. Acid ahydrides and. Acetic Acid Ethyl Ester.
From pdfprof.com
acetylation reaction mechanism acetic anhydride Acetic Acid Ethyl Ester Chemical reaction of acetic acid and ethanol to produce an ester: Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Chemistry (openstax), cc by 4.0). The odor of vinegar. Acetic Acid Ethyl Ester.
From www.pinterest.com
Example of Acetoacetic Ester Synthesis Ester, Synthesis, Chemistry Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. Name common carboxylic acids and esters. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Chemistry (openstax), cc by 4.0). Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Chemical reaction of acetic. Acetic Acid Ethyl Ester.
From www.online-sciences.com
Physical and chemical properties of Esters, Esterification reaction and Acetic Acid Ethyl Ester It is widely produced all over the world with an estimation of more than 1 million tons per. Name common carboxylic acids and esters. Ethyl acetate is the ester of ethanol and acetic acid. Chemical reaction of acetic acid and ethanol to produce an ester: Chemistry (openstax), cc by 4.0). Esters occur widely in nature. Describe the structure and properties. Acetic Acid Ethyl Ester.
From www.dreamstime.com
Ethyl Acetate, Ethyl Ethanoate, C4H8O2 Molecule. it is Acetate Ester Acetic Acid Ethyl Ester Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Describe the structure and properties of carboxylic acids and esters. Name common carboxylic acids and esters. Esters can also be formed by deprotonating a carboxylic. It is widely produced all over the world with an estimation of more than 1 million tons per. Esters occur widely in nature.. Acetic Acid Ethyl Ester.
From dir.indiamart.com
Ethyl Acetate ethyl ethanoate Latest Price, Manufacturers & Suppliers Acetic Acid Ethyl Ester The odor of vinegar is caused by the presence of acetic acid, a. Esters can also be formed by deprotonating a carboxylic. It is widely produced all over the world with an estimation of more than 1 million tons per. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur. Acetic Acid Ethyl Ester.
From store.p212121.com
(2Cyanophenoxy)acetic acid ethyl ester CAS 39786340 P212121 Store Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. Name common carboxylic acids and esters. Chemistry (openstax), cc by 4.0). The odor of vinegar is caused by the presence of acetic acid, a. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Esters occur widely in nature. Chemical reaction of acetic. Acetic Acid Ethyl Ester.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Acetic Acid Ethyl Ester Name common carboxylic acids and esters. Ethyl acetate is the ester of ethanol and acetic acid. It is widely produced all over the world with an estimation of more than 1 million tons per. Esters can also be formed by deprotonating a carboxylic. Chemical reaction of acetic acid and ethanol to produce an ester: Ethyl acetate is the acetate ester. Acetic Acid Ethyl Ester.
From byjus.com
When ethyl alcohol and acetic acid mixed together in equimolecular Acetic Acid Ethyl Ester Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Describe the structure and properties of carboxylic acids and esters. Esters can also be formed by deprotonating a carboxylic. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. It is widely produced all over. Acetic Acid Ethyl Ester.
From sielc.com
1,3Dioxolane2acetic acid, 2methyl, ethyl ester SIELC Acetic Acid Ethyl Ester Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Ethyl acetate is the ester of ethanol and acetic acid. Esters occur widely in nature. Esters can also be formed by deprotonating a carboxylic. Describe the structure and properties of carboxylic acids and esters. It is widely produced all over the world with an estimation of more than. Acetic Acid Ethyl Ester.
From www.echemi.com
Buy Ethyl acetate Acetic ester Acetic ether Ethyl ester of acetic acid Acetic Acid Ethyl Ester Chemical reaction of acetic acid and ethanol to produce an ester: Ethyl acetate is the ester of ethanol and acetic acid. The odor of vinegar is caused by the presence of acetic acid, a. This can occur through the. Describe the structure and properties of carboxylic acids and esters. Acid ahydrides and carboxylic acids can also react with alcohols to. Acetic Acid Ethyl Ester.
From www.guidechem.com
2(3ethoxy4oxanyl)acetic acid ethyl ester 2104546463 wiki Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Name common carboxylic acids and esters. Esters occur widely in nature. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple. Acetic Acid Ethyl Ester.
From www.chegg.com
Solved Show the calculations that determine how much water Acetic Acid Ethyl Ester Describe the structure and properties of carboxylic acids and esters. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. It is widely produced all over the world with an estimation of more than 1 million tons per. Acid ahydrides and. Acetic Acid Ethyl Ester.
From store.p212121.com
(3Formylindol1yl)acetic acid ethyl ester CAS 27065947 Acetic Acid Ethyl Ester Esters can also be formed by deprotonating a carboxylic. Esters occur widely in nature. Chemical reaction of acetic acid and ethanol to produce an ester: It is widely produced all over the world with an estimation of more than 1 million tons per. Name common carboxylic acids and esters. This can occur through the. Ethyl acetate is the acetate ester. Acetic Acid Ethyl Ester.
From sielc.com
Acetic acid, fluoro, ethyl ester SIELC Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. This can occur through the. Describe the structure and properties of carboxylic acids and esters. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Chemistry (openstax), cc by 4.0). Acid. Acetic Acid Ethyl Ester.
From www.dreamstime.com
Ethyl Acetate, Ethyl Ethanoate, C4H8O2 Molecule. it is Acetate Ester Acetic Acid Ethyl Ester This can occur through the. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. It is widely produced all over the world with an estimation of more than 1 million tons per. Name common carboxylic acids and esters. Esters occur widely in nature. The odor of. Acetic Acid Ethyl Ester.
From www.alamy.com
Ethyl acetate, ethyl ethanoate, C4H8O2 molecule. It is acetate ester Acetic Acid Ethyl Ester Ethyl acetate is the acetate ester formed between acetic acid and ethanol. It is widely produced all over the world with an estimation of more than 1 million tons per. Esters occur widely in nature. Chemistry (openstax), cc by 4.0). Chemical reaction of acetic acid and ethanol to produce an ester: Name common carboxylic acids and esters. Ethyl acetate is. Acetic Acid Ethyl Ester.
From store.p212121.com
Chlorosulfonyl acetic acid ethyl ester CAS 55896930 P212121 Store Acetic Acid Ethyl Ester Esters can also be formed by deprotonating a carboxylic. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Name common carboxylic acids and esters. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. The odor of vinegar is caused by the presence of acetic acid, a. Describe the structure. Acetic Acid Ethyl Ester.
From chem.libretexts.org
12.4 Esters Chemistry LibreTexts Acetic Acid Ethyl Ester Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Chemistry (openstax), cc by 4.0). It is widely produced all over the world with an estimation of more than 1 million tons per. Chemical reaction of acetic acid and ethanol to produce an ester: Esters can also. Acetic Acid Ethyl Ester.
From sielc.com
Acetic acid, diethoxy, ethyl ester SIELC Acetic Acid Ethyl Ester Describe the structure and properties of carboxylic acids and esters. Chemistry (openstax), cc by 4.0). The odor of vinegar is caused by the presence of acetic acid, a. Esters occur widely in nature. Name common carboxylic acids and esters. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Chemical reaction of acetic acid and ethanol to produce. Acetic Acid Ethyl Ester.
From www.chemicalbook.com
TETRAHYDROFURAN2ACETIC ACID ETHYL ESTER(2434028) 1H NMR spectrum Acetic Acid Ethyl Ester Describe the structure and properties of carboxylic acids and esters. Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Esters occur widely in nature. Chemistry (openstax), cc by 4.0). Acid ahydrides and carboxylic acids can also react with alcohols to form esters. Acetic Acid Ethyl Ester.
From ar.inspiredpencil.com
Structure Of Ethyl Acetate Acetic Acid Ethyl Ester The odor of vinegar is caused by the presence of acetic acid, a. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur through the. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Acid ahydrides and carboxylic acids can also react with alcohols to form esters. Acetic Acid Ethyl Ester.
From www.penli.fi
Acetic acid ethyl ester LCMS Grade Penli Acetic Acid Ethyl Ester Ethyl acetate is the ester of ethanol and acetic acid. The odor of vinegar is caused by the presence of acetic acid, a. Esters occur widely in nature. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Name common carboxylic. Acetic Acid Ethyl Ester.
From www.sielc.com
Acetic acid, 2(dimethylamino)ethyl ester SIELC Acetic Acid Ethyl Ester Chemistry (openstax), cc by 4.0). Esters can also be formed by deprotonating a carboxylic. This can occur through the. Chemical reaction of acetic acid and ethanol to produce an ester: Esters occur widely in nature. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be. Acetic Acid Ethyl Ester.
From www.guidechem.com
2(2cyano4formyl5hydroxyphenyl)acetic acid ethyl ester 180704490 Acetic Acid Ethyl Ester This can occur through the. Esters occur widely in nature. Chemistry (openstax), cc by 4.0). Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Ethyl acetate is the ester of ethanol and acetic acid. Ethyl acetate is the acetate ester formed between acetic acid and ethanol.. Acetic Acid Ethyl Ester.
From en.wikipedia.org
Ethyl acetate Wikipedia Acetic Acid Ethyl Ester Chemistry (openstax), cc by 4.0). Chemical reaction of acetic acid and ethanol to produce an ester: Esters can also be formed by deprotonating a carboxylic. Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited. Acetic Acid Ethyl Ester.
From sielc.com
Indole3acetic acid ethyl ester SIELC Technologies Acetic Acid Ethyl Ester Chemical reaction of acetic acid and ethanol to produce an ester: Esters can also be formed by deprotonating a carboxylic. Chemistry (openstax), cc by 4.0). This can occur through the. Esters occur widely in nature. Describe the structure and properties of carboxylic acids and esters. Ethyl acetate is the acetate ester formed between acetic acid and ethanol. Name common carboxylic. Acetic Acid Ethyl Ester.
From www.sielc.com
Acetic acid, phenoxy, ethyl ester SIELC Acetic Acid Ethyl Ester Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. The odor of vinegar is caused by the presence of acetic acid, a. Esters can also be formed by deprotonating a carboxylic. It is widely produced all over the world with an estimation of more than 1 million tons per. Esters occur widely. Acetic Acid Ethyl Ester.