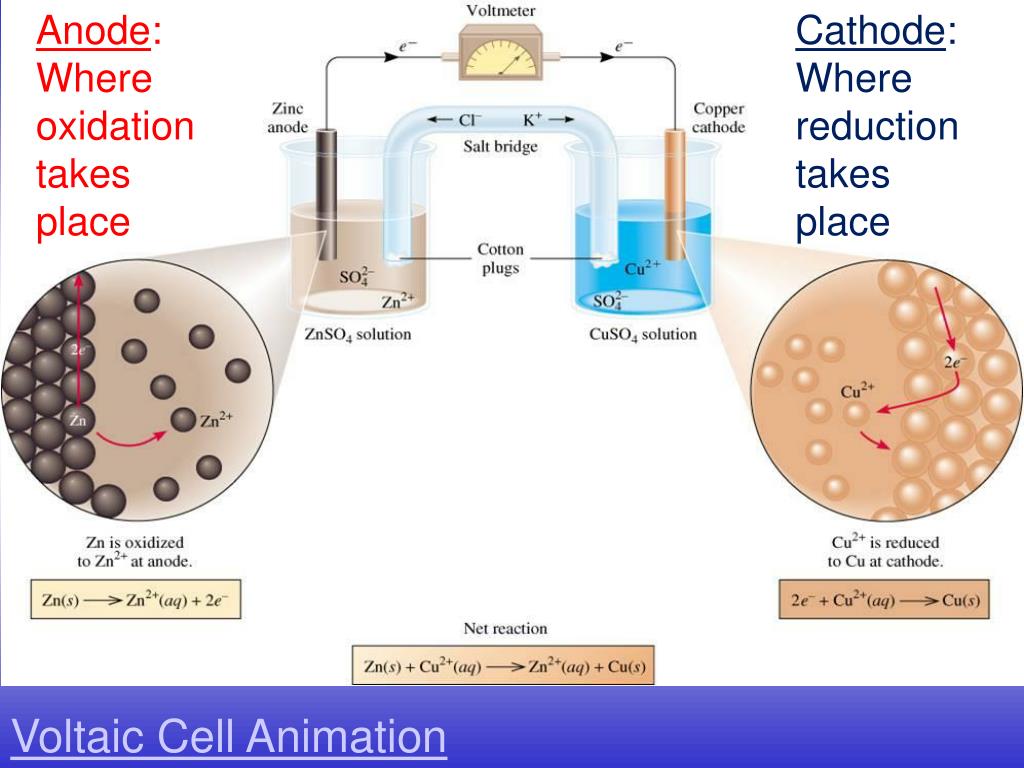
Electrochemistry Oxidation . Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemistry is the study of chemical processes that cause electrons to move. Notice that these are exactly the opposite of the oxygen definitions (#1). Oxidation is the loss of hydrogen. This movement of electrons is called.
from www.slideserve.com
Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. This movement of electrons is called. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Oxidation is the loss of hydrogen. A redox reaction occurs when electrons are transferred. Notice that these are exactly the opposite of the oxygen definitions (#1).
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation
Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. A redox reaction occurs when electrons are transferred. Electrochemistry is the study of chemical processes that cause electrons to move. Notice that these are exactly the opposite of the oxygen definitions (#1). Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. This movement of electrons is called. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Oxidation is the loss of hydrogen.
From www.researchgate.net
The schematic diagram for the electrochemical oxidation process of AA Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Notice that these are exactly the opposite of the oxygen definitions (#1). This movement of electrons is called. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Oxidation is the loss of hydrogen. A. Electrochemistry Oxidation.
From rjalili.com
Protons from H2 for Ammonia Synthesis A Promising Electrochemical Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Oxidation is the loss of hydrogen. A redox reaction occurs when electrons are transferred. Electrochemistry is the study of chemical processes that cause electrons to move. Notice that these are exactly the opposite of the oxygen definitions (#1). During an electrolysis, oxidation. Electrochemistry Oxidation.
From saylordotorg.github.io
Electrochemistry Electrochemistry Oxidation Reduction is the gain of hydrogen. Oxidation is the loss of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. A redox reaction occurs when electrons are transferred. Electrochemistry is the study of chemical processes that cause electrons to move. Notice that these are exactly the opposite of the oxygen definitions. Electrochemistry Oxidation.
From klayyqrgm.blob.core.windows.net
Electrochemical Oxidation Products at James Szeto blog Electrochemistry Oxidation Notice that these are exactly the opposite of the oxygen definitions (#1). A redox reaction occurs when electrons are transferred. This movement of electrons is called. Reduction is the gain of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemical organic oxidation reactions are highly appealing because protons are often. Electrochemistry Oxidation.
From sonlasopa454.weebly.com
Modern water treatment by electrochemical oxidation sonlasopa Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Notice that these are exactly the opposite of the oxygen definitions (#1). A redox reaction occurs when electrons are transferred. Electrochemistry is the study of. Electrochemistry Oxidation.
From www.slideserve.com
PPT Oxidation, Reduction and Electrochemistry PowerPoint Presentation Electrochemistry Oxidation Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. This movement of electrons is called. Oxidation is the loss of hydrogen. A redox reaction occurs when electrons are transferred. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemistry is the study. Electrochemistry Oxidation.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Electrochemistry Oxidation This movement of electrons is called. Reduction is the gain of hydrogen. A redox reaction occurs when electrons are transferred. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Electrochemistry is the study of chemical processes that cause electrons to move. Oxidation is the loss of hydrogen. During an electrolysis, oxidation. Electrochemistry Oxidation.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Electrochemistry Oxidation During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Reduction is the gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions (#1). A redox reaction occurs when electrons are. Electrochemistry Oxidation.
From esemag.com
Electrooxidation shows promise for treating high strength ethylene Electrochemistry Oxidation A redox reaction occurs when electrons are transferred. Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding. Electrochemistry Oxidation.
From saylordotorg.github.io
Electrochemistry Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. A redox reaction occurs when electrons are transferred. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. This movement of electrons is. Electrochemistry Oxidation.
From onlinelibrary.wiley.com
Electrochemical Oxidation of Alcohols Using Halogen Mediators Electrochemistry Oxidation Oxidation is the loss of hydrogen. Reduction is the gain of hydrogen. Electrochemistry is the study of chemical processes that cause electrons to move. A redox reaction occurs when electrons are transferred. Notice that these are exactly the opposite of the oxygen definitions (#1). This movement of electrons is called. Electrochemical organic oxidation reactions are highly appealing because protons are. Electrochemistry Oxidation.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. Oxidation is the loss of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively. Electrochemistry Oxidation.
From saylordotorg.github.io
Electrochemistry Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. A redox reaction occurs when electrons are transferred. Oxidation is the loss of hydrogen. Reduction is the gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions. Electrochemistry Oxidation.
From saylordotorg.github.io
Electrochemistry Electrochemistry Oxidation A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding. Electrochemistry Oxidation.
From pubs.acs.org
Electrochemical Oxidation Processes for the Treatment of Organic Electrochemistry Oxidation Reduction is the gain of hydrogen. A redox reaction occurs when electrons are transferred. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Notice that these are exactly the opposite of the oxygen definitions (#1). Oxidation is the loss of hydrogen. Electrochemistry is the study of chemical processes that cause electrons to. Electrochemistry Oxidation.
From www.youtube.com
19 Electrochemistry Oxidation Reduction Reactions YouTube Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. A redox reaction occurs when electrons are transferred. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Reduction is the gain of hydrogen. This movement of electrons is called. Oxidation is the loss of hydrogen. Notice that these are. Electrochemistry Oxidation.
From www.researchgate.net
The electrooxidation process involves electron (e⁻) transfer and in Electrochemistry Oxidation Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. A redox reaction occurs when electrons are transferred. Electrochemical organic oxidation reactions are. Electrochemistry Oxidation.
From www.researchgate.net
The electrochemical oxidation (a) and reduction (b) of GC4000 by CV Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called. A redox reaction occurs when electrons are transferred. Oxidation is the loss of hydrogen. Notice that these are exactly the opposite of the oxygen. Electrochemistry Oxidation.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemical organic oxidation reactions are. Electrochemistry Oxidation.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemistry Oxidation Notice that these are exactly the opposite of the oxygen definitions (#1). Reduction is the gain of hydrogen. Oxidation is the loss of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. During an electrolysis, oxidation occurs at the positively charged anode,. Electrochemistry Oxidation.
From www.studocu.com
Electrochemistry OxidationReduction Reactions { Electrochemistry Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. This movement of electrons is called. Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Oxidation is the loss of hydrogen. Reduction. Electrochemistry Oxidation.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Electrochemistry Oxidation A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Oxidation is the loss of hydrogen. This movement of electrons is called. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemistry is the study. Electrochemistry Oxidation.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Notice that these are exactly the opposite of the oxygen definitions (#1). A redox reaction occurs when electrons are transferred. Oxidation is the loss. Electrochemistry Oxidation.
From socratic.org
What is a voltaic cell? + Example Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Reduction is the gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions (#1). During an electrolysis, oxidation occurs at. Electrochemistry Oxidation.
From pubs.rsc.org
Critical assessment of advanced oxidation processes and bio Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. Notice that these are exactly the opposite of the oxygen definitions (#1). This movement of electrons is called. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. During an electrolysis, oxidation. Electrochemistry Oxidation.
From loebepedm.blob.core.windows.net
Electrochemical Activity Of at Desiree Kirklin blog Electrochemistry Oxidation Reduction is the gain of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Oxidation is the loss of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. Electrochemistry is the study of. Electrochemistry Oxidation.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrochemistry Oxidation During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Notice that these are exactly the opposite of the oxygen definitions (#1). Oxidation is the loss of hydrogen. Reduction is the gain of hydrogen. Electrochemistry is the study of chemical processes that cause electrons to move. A redox reaction occurs when electrons are. Electrochemistry Oxidation.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Electrochemistry Oxidation A redox reaction occurs when electrons are transferred. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. This movement of electrons is called. Reduction is the gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemical organic oxidation reactions are highly appealing because protons are often. Electrochemistry Oxidation.
From study.com
Electrochemical Cells and Electrochemistry Lesson Electrochemistry Oxidation Oxidation is the loss of hydrogen. Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. This movement of electrons is called. A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are. Electrochemistry Oxidation.
From chemistry.stackexchange.com
physical chemistry Electrochemistry (Spontaneous Reactions Electrochemistry Oxidation Reduction is the gain of hydrogen. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Electrochemistry is the study of chemical processes that cause electrons to move. Oxidation is the loss of hydrogen. This. Electrochemistry Oxidation.
From klayyqrgm.blob.core.windows.net
Electrochemical Oxidation Products at James Szeto blog Electrochemistry Oxidation Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Oxidation is the loss of. Electrochemistry Oxidation.
From www.scienceabc.com
Scientific Accuracy Of The Battery Scene In Breaking Bad Can It Really Electrochemistry Oxidation Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A redox reaction occurs when electrons are transferred. Reduction is the gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemistry is the study of chemical processes that cause electrons to move. During an electrolysis, oxidation. Electrochemistry Oxidation.
From pubs.rsc.org
Electrochemical oxidation of styrene to benzaldehyde by discrimination Electrochemistry Oxidation Reduction is the gain of hydrogen. Oxidation is the loss of hydrogen. This movement of electrons is called. A redox reaction occurs when electrons are transferred. Notice that these are exactly the opposite of the oxygen definitions (#1). Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. Electrochemistry is the study. Electrochemistry Oxidation.
From www.pinterest.co.uk
Electrochemical series conversation trick to rememberElectrochemistry Electrochemistry Oxidation A redox reaction occurs when electrons are transferred. Notice that these are exactly the opposite of the oxygen definitions (#1). Oxidation is the loss of hydrogen. This movement of electrons is called. Electrochemistry is the study of chemical processes that cause electrons to move. Reduction is the gain of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are. Electrochemistry Oxidation.
From www.youtube.com
Difference between Oxidation and Reduction Redox reaction Class 11 Electrochemistry Oxidation This movement of electrons is called. During an electrolysis, oxidation occurs at the positively charged anode, while reduction occurs at the negatively charged. Electrochemistry is the study of chemical processes that cause electrons to move. Oxidation is the loss of hydrogen. Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable. A. Electrochemistry Oxidation.