Hard Water Results From Relatively High Concentrations Of Dissolved . Temporary hardness is due to the presence of. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Water is considered hard when it has a relatively high concentration of calcium and magnesium. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Hard water is high in. Hard water received this name because it. Soft water contains lower concentrations of these minerals. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water.
from www.energy.gov
Water is considered hard when it has a relatively high concentration of calcium and magnesium. Temporary hardness is due to the presence of. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Soft water contains lower concentrations of these minerals. Hard water is high in. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Hard water received this name because it. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water.
Understanding and Dealing With Hard Water Department of Energy
Hard Water Results From Relatively High Concentrations Of Dissolved Soft water contains lower concentrations of these minerals. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is high in. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Soft water contains lower concentrations of these minerals. Hard water received this name because it. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Temporary hardness is due to the presence of.
From www.wikihow.com
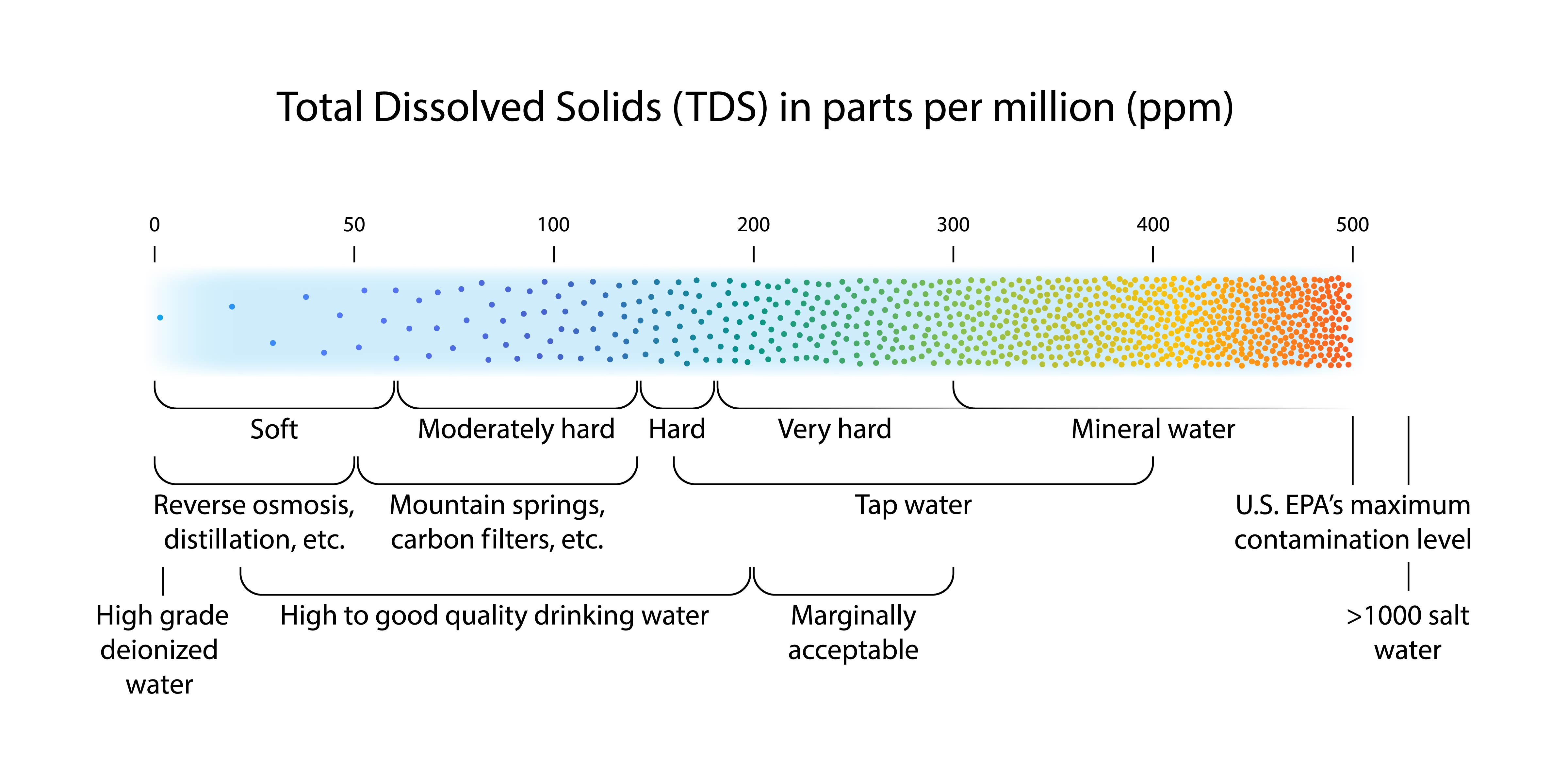
How to Calculate Total Dissolved Solids 10 Steps (with Pictures) Hard Water Results From Relatively High Concentrations Of Dissolved Hard water is high in. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Temporary hardness is due to the presence of. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Soft water contains lower concentrations of these minerals. The relatively. Hard Water Results From Relatively High Concentrations Of Dissolved.
From testaqua.com
TDS Results Chart Bottled Water Tests Hard Water Results From Relatively High Concentrations Of Dissolved Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Soft water contains lower concentrations of these minerals. The relatively high concentrations of these ions can saturate the solution and consequently cause the. Hard Water Results From Relatively High Concentrations Of Dissolved.
From waterfilterguru.com
What Is TDS in Water and What Does it Indicate? Hard Water Results From Relatively High Concentrations Of Dissolved Temporary hardness is due to the presence of. Hard water received this name because it. Water is considered hard when it has a relatively high concentration of calcium and magnesium. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Hard water is water that contains dissolved minerals such as calcium, magnesium,. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Time series of dissolved oxygen concentrations in the surface water Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Hard water received this name because it. Hard water is high in. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Dissolved oxygen concentrations in nearbottom water measured in the Hard Water Results From Relatively High Concentrations Of Dissolved As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Hard water received this name because it. Hard water is high in. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The relatively high concentrations of these ions can saturate the solution and consequently cause the. Hard Water Results From Relatively High Concentrations Of Dissolved.
From books.gw-project.org
Natural Chemical Constituents in Groundwater Groundwater in Our Water Hard Water Results From Relatively High Concentrations Of Dissolved Hard water received this name because it. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Hard water is high in. Temporary hardness is due to the presence of. Soft water contains lower concentrations of these minerals.. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.chegg.com
Solved TABLE P15.5 Dissolved oxygen concentration in water Hard Water Results From Relatively High Concentrations Of Dissolved An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Water is considered hard when it has a relatively high concentration of calcium and magnesium. Soft water contains lower concentrations of these minerals. Hard water is high in. As a result, the groundwater attains a high concentration of. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Dissolved concentrations of Cu, Ni, Cd, and As in water surface in five Hard Water Results From Relatively High Concentrations Of Dissolved An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Soft water contains lower concentrations of these minerals. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Hard water received this. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Plot of dissolved silica concentration versus pH in groundwater samples Hard Water Results From Relatively High Concentrations Of Dissolved Temporary hardness is due to the presence of. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Hard water is high in. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Water is considered hard when it has a relatively high. Hard Water Results From Relatively High Concentrations Of Dissolved.
From quenchwater.com
TDS Information Sheet Total Dissolved Solids Quench Water Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water received this name because it. Hard water is high in. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. As a result, the groundwater. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Hard Water Results From Relatively High Concentrations Of Dissolved Water is considered hard when it has a relatively high concentration of calcium and magnesium. Hard water is high in. Hard water received this name because it. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Temporary hardness is due to the presence of. Soft water contains lower concentrations of these. Hard Water Results From Relatively High Concentrations Of Dissolved.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Hard water received this name because it. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift. Hard Water Results From Relatively High Concentrations Of Dissolved.
From courses.lumenlearning.com
The Dissolution Process Chemistry for Majors Hard Water Results From Relatively High Concentrations Of Dissolved Soft water contains lower concentrations of these minerals. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Hard water is high in. Water is considered hard when it has a relatively high concentration of calcium and magnesium. Temporary hardness is due to the. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Typical relationships between dissolved concentrations and discharge Hard Water Results From Relatively High Concentrations Of Dissolved Soft water contains lower concentrations of these minerals. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. The relatively high concentrations of these ions can saturate the solution and consequently cause the. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Fig. S2. Pore water concentrations of dissolved (A) sulfate (mM), (B Hard Water Results From Relatively High Concentrations Of Dissolved The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Soft water contains lower concentrations of these minerals. Hard water is high in. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Hard water received. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Relation between pH and dissolved methane concentration in groundwater Hard Water Results From Relatively High Concentrations Of Dissolved Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Soft water contains lower concentrations of these minerals. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The simple definition of. Hard Water Results From Relatively High Concentrations Of Dissolved.
From serc.carleton.edu
The Chemistry of Natural Waters Hard Water Results From Relatively High Concentrations Of Dissolved Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is high in. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The simple definition. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Evolution of dissolved CO 2 concentration with different flow Hard Water Results From Relatively High Concentrations Of Dissolved Water is considered hard when it has a relatively high concentration of calcium and magnesium. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is high in. Hard. Hard Water Results From Relatively High Concentrations Of Dissolved.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Water is considered hard when it has a relatively high concentration of calcium and magnesium. Water hardness. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.energy.gov
Understanding and Dealing With Hard Water Department of Energy Hard Water Results From Relatively High Concentrations Of Dissolved Temporary hardness is due to the presence of. Hard water is high in. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Soft water contains lower concentrations of these minerals. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water received this name because it.. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Changes in the concentration of dissolved oxygen in the water in the Hard Water Results From Relatively High Concentrations Of Dissolved Soft water contains lower concentrations of these minerals. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Hard water is high in. Temporary hardness is due to the presence of. Hard water received this name because it. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Hard Water Results From Relatively High Concentrations Of Dissolved Hard water received this name because it. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Temporary hardness is due to the presence of. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The relatively high concentrations of these ions can saturate the solution and consequently. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Correlation between chloride concentrations and total dissolved solids Hard Water Results From Relatively High Concentrations Of Dissolved Hard water is high in. Temporary hardness is due to the presence of. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. The simple definition of water hardness. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.pinterest.ca
What is Hard Water? Hard water is water containing high concentrations Hard Water Results From Relatively High Concentrations Of Dissolved Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Water is considered hard when. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Hard Water Results From Relatively High Concentrations Of Dissolved Hard water received this name because it. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. The simple definition of water hardness is the amount of dissolved calcium and. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.kgs.ku.edu
KGSGround Water Series 9TriState Region Ground WatersChemical Hard Water Results From Relatively High Concentrations Of Dissolved Hard water received this name because it. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Soft water contains lower concentrations of these minerals. Water hardness is a function of quanta of. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Mean (n = 3) values of pH, and concentration of dissolved O 2 , SO 4 Hard Water Results From Relatively High Concentrations Of Dissolved Hard water is high in. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. The simple definition of water hardness is the amount of dissolved calcium and magnesium in. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.youtube.com
Total Dissolved Solids (TDS) Water Chemistry Basics Course YouTube Hard Water Results From Relatively High Concentrations Of Dissolved Water is considered hard when it has a relatively high concentration of calcium and magnesium. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. Hard water is water that contains dissolved minerals such as calcium, magnesium, iron and manganese. Hard water received this. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Hard Water Results From Relatively High Concentrations Of Dissolved Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Hard water received this name because it. Soft water contains lower concentrations of these minerals. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. The simple definition of water hardness is the amount of dissolved calcium. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Plot of dissolved silica concentration versus pH in groundwater samples Hard Water Results From Relatively High Concentrations Of Dissolved Soft water contains lower concentrations of these minerals. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. The simple definition of water hardness is the amount of dissolved calcium and magnesium in. Hard Water Results From Relatively High Concentrations Of Dissolved.
From webmis.highland.cc.il.us
Solution Concentrations Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Hard water is water that contains. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Dissolved Oxygen Concentration values of the good / moderate boundaries Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Water is considered hard when it has a relatively high concentration of calcium and magnesium. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. The relatively high concentrations of these ions can saturate the. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.wahlwater.com
Total Dissolved Solids Jeff Wahl Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. An alternative measure of hardness is total dissolved solids (tds), which is a measure of the total concentration of ions in water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. Soft water contains lower concentrations of. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Diurnal variations of dissolved oxygen concentration in lakewater Hard Water Results From Relatively High Concentrations Of Dissolved The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water received this name because it. Hard water is high in. As a result, the groundwater attains a high concentration of dissolved constituents and is characterized as hard water. The relatively high concentrations of these ions can saturate the solution and consequently. Hard Water Results From Relatively High Concentrations Of Dissolved.
From www.researchgate.net
Concentrations of dissolved oxygen (A) and seawater temperature (B Hard Water Results From Relatively High Concentrations Of Dissolved Hard water received this name because it. Water is considered hard when it has a relatively high concentration of calcium and magnesium. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Water hardness is a function of quanta of dissolved calcium and magnesium ions in water. An alternative measure of hardness is. Hard Water Results From Relatively High Concentrations Of Dissolved.