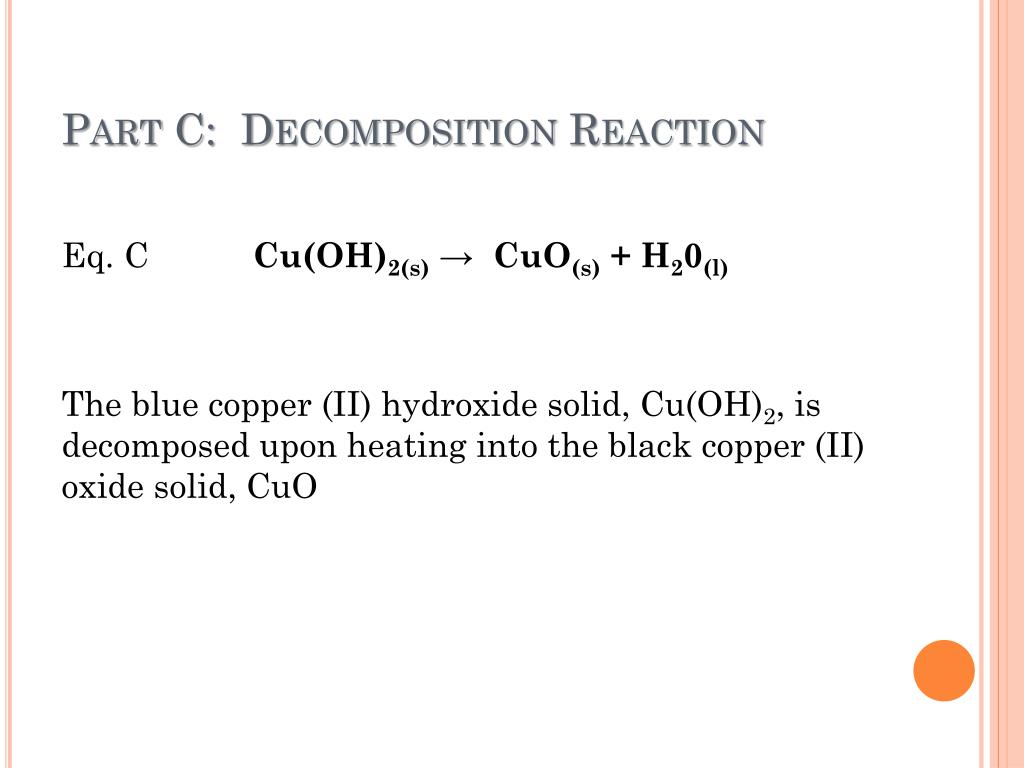
Decomposition Of Copper Ii Nitrate . Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. What is the relationship between the extent of decomposition and the. 2x (no3)2 (s) 2xo (s) + 4no2 (g). If 1.0 mole of copper(ii) nitrate. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. When heated, copper nitrate decomposes into copper oxide cuo,. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Explore the factors that affect the thermal stability of these compounds. The general equation for the decomposition of group 2 nitrates is: Copper nitrate cu no 3 2 is a blue crystalline solid.
from www.slideserve.com
2x (no3)2 (s) 2xo (s) + 4no2 (g). Copper nitrate cu no 3 2 is a blue crystalline solid. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Explore the factors that affect the thermal stability of these compounds. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. When heated, copper nitrate decomposes into copper oxide cuo,. The general equation for the decomposition of group 2 nitrates is: Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated.
PPT Chemical Reactions Copper Reactions PowerPoint Presentation
Decomposition Of Copper Ii Nitrate What is the relationship between the extent of decomposition and the. The general equation for the decomposition of group 2 nitrates is: The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. When heated, copper nitrate decomposes into copper oxide cuo,. Copper nitrate cu no 3 2 is a blue crystalline solid. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. Explore the factors that affect the thermal stability of these compounds. What is the relationship between the extent of decomposition and the. 2x (no3)2 (s) 2xo (s) + 4no2 (g). If 1.0 mole of copper(ii) nitrate.
From www.researchgate.net
(PDF) Mechanism of thermal of hydrated copper nitrate in Decomposition Of Copper Ii Nitrate The general equation for the decomposition of group 2 nitrates is: If 1.0 mole of copper(ii) nitrate. Copper nitrate cu no 3 2 is a blue crystalline solid. Explore the factors that affect the thermal stability of these compounds. 2x (no3)2 (s) 2xo (s) + 4no2 (g). X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Learn. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] 4. Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. 2x (no3)2 (s) 2xo (s) + 4no2 (g). Copper nitrate cu no 3 2 is a blue crystalline solid. If 1.0 mole of copper(ii) nitrate. The general. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved What mass of copper (II) nitrate, Cu(NO_3)_2, must be Decomposition Of Copper Ii Nitrate The general equation for the decomposition of group 2 nitrates is: What is the relationship between the extent of decomposition and the. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Copper nitrate cu no 3 2 is a blue crystalline solid. When heated, copper nitrate decomposes into copper oxide cuo,. Learn how the carbonates and. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate Explore the factors that affect the thermal stability of these compounds. What is the relationship between the extent of decomposition and the. The general equation for the decomposition of group 2 nitrates is: Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Copper nitrate cu no 3 2 is. Decomposition Of Copper Ii Nitrate.
From www.numerade.com
SOLVED When reacting copper(II) nitrate and sodium bicarbonate, you Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. When heated, copper nitrate decomposes into copper oxide cuo,. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. If 1.0 mole of copper(ii) nitrate.. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved 8 (c) The equation for the of Decomposition Of Copper Ii Nitrate Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. If 1.0 mole of copper(ii) nitrate. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved Copper(II) nitrate on heating according to Decomposition Of Copper Ii Nitrate Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. If 1.0 mole of copper(ii) nitrate. When heated, copper nitrate decomposes into copper oxide cuo,. Copper nitrate cu no 3 2 is a blue crystalline solid. What is the relationship between the extent of decomposition and the. (iii) copper( ii) nitrate decomposes to form copper(ii). Decomposition Of Copper Ii Nitrate.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Decomposition Of Copper Ii Nitrate When heated, copper nitrate decomposes into copper oxide cuo,. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Explore the factors that affect the thermal stability of these compounds. If 1.0 mole of copper(ii) nitrate. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. X. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] 4. Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. What is the relationship between the extent of decomposition and the. Explore the factors that affect the thermal stability of these compounds. Learn how the carbonates and nitrates. Decomposition Of Copper Ii Nitrate.
From www.researchgate.net
Thermal of copper (II) nitrate trihydrate a DTA/TG Decomposition Of Copper Ii Nitrate Copper nitrate cu no 3 2 is a blue crystalline solid. When heated, copper nitrate decomposes into copper oxide cuo,. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating.. Decomposition Of Copper Ii Nitrate.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Decomposition Of Copper Ii Nitrate When heated, copper nitrate decomposes into copper oxide cuo,. If 1.0 mole of copper(ii) nitrate. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. 2x (no3)2 (s) 2xo (s) + 4no2 (g). The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. Learn how the carbonates. Decomposition Of Copper Ii Nitrate.
From www.youtube.com
How to Balance Cu(NO3)2 = CuO + NO2 + O2 Copper (II) nitrate Decomposition Of Copper Ii Nitrate Explore the factors that affect the thermal stability of these compounds. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. 2x (no3)2 (s) 2xo (s) + 4no2 (g). When heated, copper nitrate decomposes into copper oxide cuo,. Copper nitrate cu no 3 2 is a blue crystalline solid. The. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] 4. Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate When heated, copper nitrate decomposes into copper oxide cuo,. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. 2x (no3)2 (s) 2xo (s) + 4no2 (g). Learn how the carbonates and nitrates of. Decomposition Of Copper Ii Nitrate.
From www.researchgate.net
Scheme 8. Thermal mechanism of copper(II) nitrate Decomposition Of Copper Ii Nitrate The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. When heated, copper nitrate decomposes into copper oxide cuo,. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. 2x (no3)2 (s) 2xo (s) + 4no2 (g). If 1.0 mole of copper(ii) nitrate. Learn. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved 38 Copper nitrate when heated according to Decomposition Of Copper Ii Nitrate Copper nitrate cu no 3 2 is a blue crystalline solid. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. Explore the factors that affect the thermal stability of these compounds. When heated, copper nitrate decomposes into copper oxide cuo,. X (no3)2 (s) xo (s) + 2no2 (g). Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate 2x (no3)2 (s) 2xo (s) + 4no2 (g). Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. When heated, copper nitrate. Decomposition Of Copper Ii Nitrate.
From www.bartleby.com
Answered Question 5 Copper(II) nitrate… bartleby Decomposition Of Copper Ii Nitrate The general equation for the decomposition of group 2 nitrates is: Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. The thermal. Decomposition Of Copper Ii Nitrate.
From brainly.com
ΕΛΑ 1 a C Copper(II) nitrate on heating. The reaction is Decomposition Of Copper Ii Nitrate Copper nitrate cu no 3 2 is a blue crystalline solid. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. What is the relationship between the extent of decomposition and the. The general equation for the decomposition. Decomposition Of Copper Ii Nitrate.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Decomposition Of Copper Ii Nitrate The general equation for the decomposition of group 2 nitrates is: Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. 2x (no3)2 (s) 2xo (s) + 4no2 (g). The thermal decomposition of group 2. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved Copper(II) nitrate on heating according to Decomposition Of Copper Ii Nitrate Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. When heated, copper nitrate decomposes into copper oxide cuo,. The general equation for the decomposition of group 2 nitrates is: Explore the factors that affect the thermal stability of these compounds. If 1.0 mole of copper(ii) nitrate. X (no3)2 (s). Decomposition Of Copper Ii Nitrate.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2653 Science Photo Library Decomposition Of Copper Ii Nitrate Copper nitrate cu no 3 2 is a blue crystalline solid. What is the relationship between the extent of decomposition and the. (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. When heated, copper nitrate decomposes into copper oxide cuo,. 2x (no3)2 (s) 2xo (s) + 4no2 (g). Copper(ii) nitrate decomposes upon heating to form copper(ii). Decomposition Of Copper Ii Nitrate.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For The Of Copper Ii Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. When heated, copper nitrate decomposes into copper oxide cuo,. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and. Decomposition Of Copper Ii Nitrate.
From www.slideshare.net
reaction Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. Copper nitrate cu no 3 2 is a blue crystalline solid. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. The. Decomposition Of Copper Ii Nitrate.
From www.semanticscholar.org
Figure 1 from Mechanism of thermal of hydrated copper Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. If 1.0 mole of copper(ii) nitrate. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. Explore the factors that affect the thermal stability of these compounds. Copper nitrate cu no 3 2 is a blue crystalline. Decomposition Of Copper Ii Nitrate.
From www.chegg.com
Solved 4. Copper(II) nitrate on heating according Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. The thermal decomposition of group 2 nitrates and carbonates differs in both. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] 4. Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate The general equation for the decomposition of group 2 nitrates is: Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. If 1.0 mole of copper(ii) nitrate. What is the relationship between the extent of decomposition and the. The thermal decomposition of group 2 nitrates and carbonates differs in both. Decomposition Of Copper Ii Nitrate.
From www.youtube.com
Thermal of Copper(II) Nitrate YouTube Decomposition Of Copper Ii Nitrate 2x (no3)2 (s) 2xo (s) + 4no2 (g). Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. If 1.0 mole of copper(ii) nitrate. Learn how the carbonates and nitrates of the group 2. Decomposition Of Copper Ii Nitrate.
From www.coursehero.com
[Solved] 4. Copper(II) nitrate on heating according to the Decomposition Of Copper Ii Nitrate Copper nitrate cu no 3 2 is a blue crystalline solid. What is the relationship between the extent of decomposition and the. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. 2x (no3)2 (s) 2xo (s) + 4no2 (g). Explore the factors that affect the thermal stability of. Decomposition Of Copper Ii Nitrate.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Decomposition Of Copper Ii Nitrate (iii) copper( ii) nitrate decomposes to form copper(ii) oxide, nitrogen dioxide and oxygen. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. The thermal decomposition of group 2 nitrates and carbonates differs in both the products formed and the conditions required for decomposition. The general equation for the decomposition. Decomposition Of Copper Ii Nitrate.
From pubs.rsc.org
Spectroscopy and photochemistry of copper nitrate clusters Physical Decomposition Of Copper Ii Nitrate If 1.0 mole of copper(ii) nitrate. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. What is the relationship between the extent of decomposition and the. Copper nitrate cu no 3 2 is a blue crystalline solid. Explore the factors that affect the thermal stability of these compounds. X. Decomposition Of Copper Ii Nitrate.
From www.researchgate.net
What is the green intermediate product of thermal of Decomposition Of Copper Ii Nitrate Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. If 1.0 mole of copper(ii) nitrate. Explore the factors that affect the thermal stability of these compounds. (iii) copper( ii) nitrate decomposes to form copper(ii). Decomposition Of Copper Ii Nitrate.
From www.youtube.com
How to Write the Formula for Copper (II) nitrate YouTube Decomposition Of Copper Ii Nitrate If 1.0 mole of copper(ii) nitrate. 2x (no3)2 (s) 2xo (s) + 4no2 (g). X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Learn how the carbonates and nitrates of the group 2 elements decompose to give metal oxides and gases when heated. Explore the factors that affect the thermal stability of these compounds. (iii) copper( ii). Decomposition Of Copper Ii Nitrate.
From www.researchgate.net
Thermal of copper (II) nitrate trihydrate a DTA/TG Decomposition Of Copper Ii Nitrate Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. Explore the factors that affect the thermal stability of these compounds. When heated, copper nitrate decomposes into copper oxide cuo,. The general equation for the decomposition of group 2 nitrates is: If 1.0 mole of copper(ii) nitrate. (iii) copper( ii) nitrate decomposes to form copper(ii). Decomposition Of Copper Ii Nitrate.
From www.youtube.com
COPPER (II) NITRATE THE COMPLETE GUIDE YouTube Decomposition Of Copper Ii Nitrate What is the relationship between the extent of decomposition and the. Copper(ii) nitrate decomposes upon heating to form copper(ii) oxide, nitrogen dioxide gas, and oxygen gas. Learn how the carbonates and nitrates of the group 2 elements (beryllium, magnesium, calcium, strontium and barium) decompose by heating. The thermal decomposition of group 2 nitrates and carbonates differs in both the products. Decomposition Of Copper Ii Nitrate.
From www.numerade.com
SOLVED 13 Write a chemical equation for the reaction of zinc metal Decomposition Of Copper Ii Nitrate If 1.0 mole of copper(ii) nitrate. When heated, copper nitrate decomposes into copper oxide cuo,. X (no3)2 (s) xo (s) + 2no2 (g) + ½o2 (g) or. Explore the factors that affect the thermal stability of these compounds. What is the relationship between the extent of decomposition and the. Learn how the carbonates and nitrates of the group 2 elements. Decomposition Of Copper Ii Nitrate.