Chloride Ion Acid Formula . Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: What is the formula for barium chloride? \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges.
from www.thesciencehive.co.uk
\[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a.
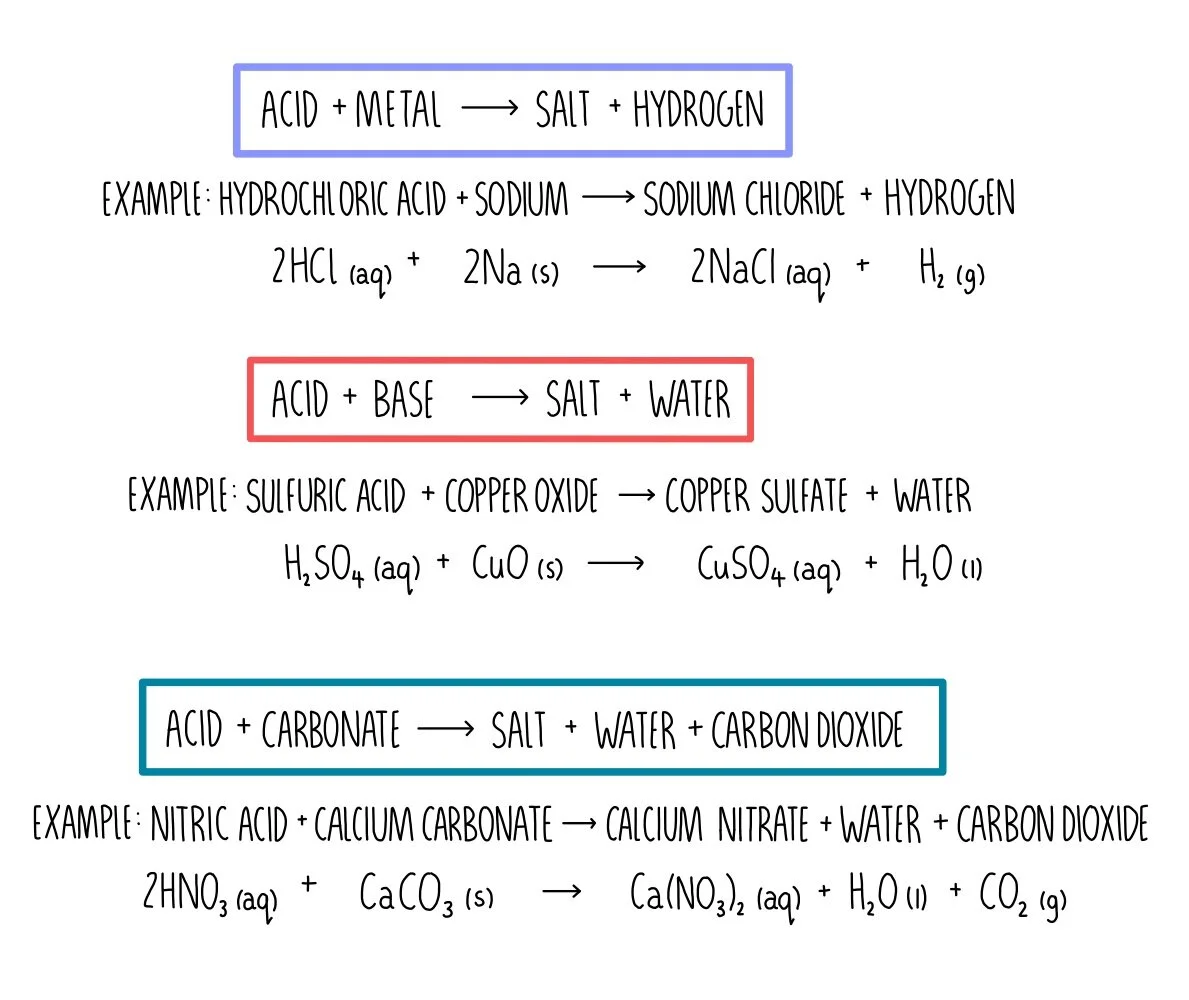
Acids, Bases and Salt Preparations (GCSE) — the science hive
Chloride Ion Acid Formula A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each. Chloride Ion Acid Formula.
From chemistrytalk.org
Carboxylic Acid Derivatives and their Reactions ChemTalk Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion. Chloride Ion Acid Formula.
From es.wikihow.com
Cómo escribir una ecuación iónica neta 10 Pasos Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when. Chloride Ion Acid Formula.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. What is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion. Chloride Ion Acid Formula.
From www.alamy.com
Hydrochloric Acid Stock Photos & Hydrochloric Acid Stock Images Alamy Chloride Ion Acid Formula What is the formula for barium chloride? A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. Chloride Ion Acid Formula.
From brokeasshome.com
Hydrogen Chloride Element Periodic Table Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. What is the. Chloride Ion Acid Formula.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Chloride Ion Acid Formula A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is. Chloride Ion Acid Formula.
From molekula.com
Purchase Sodium Chloride (tested according to EP) [7647145] online Chloride Ion Acid Formula What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when each sodium atom in a sample of sodium. Chloride Ion Acid Formula.
From study.com
Hydrogen Chloride vs. Hydrochloric Acid Formula, Properties Chloride Ion Acid Formula For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium. Chloride Ion Acid Formula.
From pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chloride Ion Acid Formula What is the formula for barium chloride? \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: For example, when each sodium. Chloride Ion Acid Formula.
From www.dreamstime.com
Hydrogen Chloride Molecule, Chemical Structure. Skeletal Formula. Stock Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the formula for barium chloride? Chloride ion is a chlorine. Chloride Ion Acid Formula.
From discover.hubpages.com
The Difference in the Chemistry of Hydrolysis & Hydration. HubPages Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the formula for barium chloride? For example, when each sodium. Chloride Ion Acid Formula.
From www.dreamstime.com
Hydrochloric Acid Hydrogen Chloride Molecule . it is a Corr Stock Chloride Ion Acid Formula A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. What is the formula for barium chloride? \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative. Chloride Ion Acid Formula.
From www.vecteezy.com
Sulfuric acid can be identified through a barium chloride test, which Chloride Ion Acid Formula A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each. Chloride Ion Acid Formula.
From www.priyamstudycentre.com
Sodium Chloride (NaCl) Uses, Crystal Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: What is the formula for barium chloride? \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. For example, when each sodium. Chloride Ion Acid Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when. Chloride Ion Acid Formula.
From mungfali.com
Acid Chloride Structure Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in. Chloride Ion Acid Formula.
From www.numerade.com
SOLVEDPART 4 Acids Writing = Milmes from acid Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the formula for barium chloride? A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. Chloride ion is a chlorine. Chloride Ion Acid Formula.
From www.jetpunk.com
Ionic Compounds by Formula Extreme Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in. Chloride Ion Acid Formula.
From www.youtube.com
Acyl Chloride and Acid Anhydride Reactions|AQA A Level Chemistry Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. What is the formula for barium chloride? For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom. Chloride Ion Acid Formula.
From www.flinnsci.ca
Ion Names, Formulas and Charges Chart Flinn Scientific Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. A magnesium ion has a 2+ charge, while a chlorine ion has a 1−. Chloride Ion Acid Formula.
From www.onlinemathlearning.com
Writing Ionic Equation (video lessons, examples and solutions) Chloride Ion Acid Formula What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom. Chloride Ion Acid Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. What is the. Chloride Ion Acid Formula.
From www.youtube.com
Cobalt Chloride Equilibrium // HSC Chemistry YouTube Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron. Chloride Ion Acid Formula.
From socratic.org
What is the chemical equation for HCl dissolving into water and Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. For example, when. Chloride Ion Acid Formula.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Chloride ion is a chlorine anion. Chloride Ion Acid Formula.
From chemstuff.co.uk
Acid Chlorides Chemstuff Chloride Ion Acid Formula \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. What is the formula for barium chloride? For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form. Chloride Ion Acid Formula.
From www.youtube.com
Naming Acid Chlorides With IUPAC Nomenclature YouTube Chloride Ion Acid Formula For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an.. Chloride Ion Acid Formula.
From www.dreamstime.com
Hydrogen Chloride Molecule, Chemical Structure. Skeletal Formula. Stock Chloride Ion Acid Formula What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. A magnesium ion has a 2+ charge, while a chlorine ion. Chloride Ion Acid Formula.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Chloride Ion Acid Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group.. Chloride Ion Acid Formula.
From mungfali.com
Acid Chloride Synthesis Chloride Ion Acid Formula Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. What is the formula for barium chloride? Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the. Chloride Ion Acid Formula.
From www.linstitute.net
CIE A Level Chemistry复习笔记7.5.2 Reactions of Carboxylic Acids翰林国际教育 Chloride Ion Acid Formula For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. What is the formula for barium chloride? A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining. Chloride Ion Acid Formula.
From www.slideserve.com
PPT Acid Base Chemistry An acid is a H + (proton) donor . General Chloride Ion Acid Formula What is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form. Chloride Ion Acid Formula.
From www.youtube.com
Naming Acids In Chemistry YouTube Chloride Ion Acid Formula What is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. For example, when each sodium atom in a sample of sodium. Chloride Ion Acid Formula.
From asideload7.gitlab.io
Formidable Word Equation Of Sodium Chloride Edexcel Physics Syllabus Chloride Ion Acid Formula For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Chloride Ion Acid Formula.