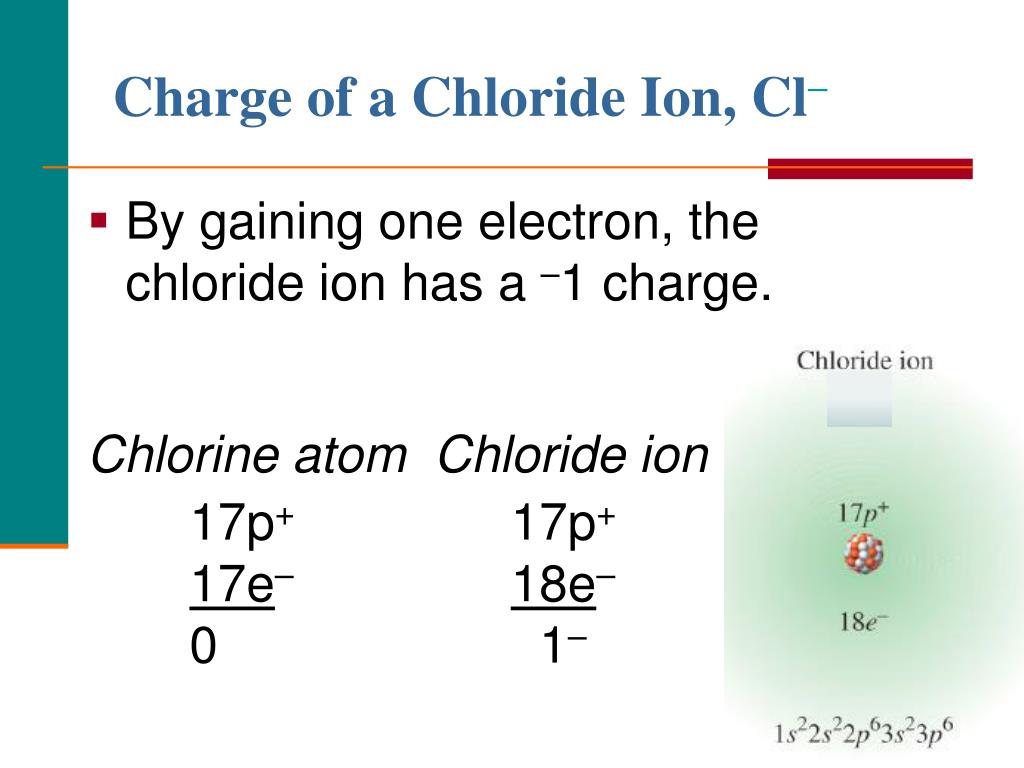
Chlorine Net Charge . When writing the formula of an ionic compound we use the lowest. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. There are two chloride ions in the formula. Net negative charge, the number of protons is less than the number of electrons.
from www.slideserve.com
Using a simple, general trend for the ionic charge for elements on the periodic table, in this. There are two chloride ions in the formula. Net negative charge, the number of protons is less than the number of electrons. When writing the formula of an ionic compound we use the lowest. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. This table shows the most common charges for atoms of the chemical elements. 93 rows updated on may 07, 2024. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
PPT The Octet Rule PowerPoint Presentation, free download ID2654700
Chlorine Net Charge This table shows the most common charges for atoms of the chemical elements. There are two chloride ions in the formula. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows updated on may 07, 2024. Net negative charge, the number of protons is less than the number of electrons. This table shows the most common charges for atoms of the chemical elements. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When writing the formula of an ionic compound we use the lowest. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows updated on may 07, 2024. Net negative charge, the number of protons is less than the number of electrons. There are two chloride ions in the formula. When these atoms gain electrons, they acquire a negative charge because they now possess. Chlorine Net Charge.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Net Charge 93 rows updated on may 07, 2024. Net negative charge, the number of protons is less than the number of electrons. When writing the formula of an ionic compound we use the lowest. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge. Chlorine Net Charge.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine Net Charge 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula. This table shows the most common charges for atoms of the chemical elements. Net negative charge, the number of protons is less than the number of electrons. When. Chlorine Net Charge.
From mungfali.com
Chlorine Orbital Diagram Chlorine Net Charge When writing the formula of an ionic compound we use the lowest. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine. Chlorine Net Charge.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Net Charge Net negative charge, the number of protons is less than the number of electrons. 93 rows updated on may 07, 2024. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. This table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic. Chlorine Net Charge.
From www.lazada.com.ph
CO MNL 500g Chlorine Powder for Disinfectant / Chlorine Granules for Chlorine Net Charge When writing the formula of an ionic compound we use the lowest. This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Net negative charge, the number of protons is less than the number of electrons. Although chlorine as. Chlorine Net Charge.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Chlorine Net Charge There are two chloride ions in the formula. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. This table shows the most common charges for atoms of the chemical elements. 93 rows updated on. Chlorine Net Charge.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Net. Chlorine Net Charge.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Net Charge This table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Net negative charge, the number of protons is less than the number of electrons. 93 rows updated on may 07, 2024. When writing the formula of an ionic compound we. Chlorine Net Charge.
From socratic.org
Question 587a9 Socratic Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Net negative charge, the number of protons is less than the number of electrons. When writing the formula of an ionic compound we use the lowest. This table shows the most common charges for atoms of the chemical elements. Although chlorine as an. Chlorine Net Charge.
From byjus.com
What is the magnitude of force between a singly charged sodium ion and Chlorine Net Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Net negative charge, the number of protons is less than the number of electrons. 93 rows updated on may 07, 2024. When writing the formula. Chlorine Net Charge.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Net Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. This table shows the most common charges for atoms of the chemical elements. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula. Net negative charge, the. Chlorine Net Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chlorine Net Charge This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Net negative charge, the number of protons is less than the number of electrons. 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic. Chlorine Net Charge.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Net Charge This table shows the most common charges for atoms of the chemical elements. There are two chloride ions in the formula. When writing the formula of an ionic compound we use the lowest. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. Using a. Chlorine Net Charge.
From giodfhmom.blob.core.windows.net
Chlorine Liquid Cost at Matthew Delgado blog Chlorine Net Charge 93 rows updated on may 07, 2024. When writing the formula of an ionic compound we use the lowest. Net negative charge, the number of protons is less than the number of electrons. There are two chloride ions in the formula. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Although. Chlorine Net Charge.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom Chlorine Net Charge 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When. Chlorine Net Charge.
From www.onlinechemistrytutor.net
chlorine001 Online Chemistry Tutor Chlorine Net Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. When writing the formula of an ionic compound we use the lowest. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula. Using a simple, general trend. Chlorine Net Charge.
From www.coursehero.com
[Solved] 3a. If an atom of chlorine has 18 negatively charged subatomic Chlorine Net Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula. This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on. Chlorine Net Charge.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Net Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. When writing the formula of an ionic compound we use the lowest. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Although chlorine as an element is. Chlorine Net Charge.
From exowfmlsz.blob.core.windows.net
Chlorine Ion Charge Formula at Angela Orlando blog Chlorine Net Charge 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. There are two chloride ions in the formula. Using a simple, general trend for the ionic charge for. Chlorine Net Charge.
From pressbooks.bccampus.ca
2.2 Chemical Bonds Douglas College Human Anatomy and Physiology I Chlorine Net Charge When writing the formula of an ionic compound we use the lowest. There are two chloride ions in the formula. This table shows the most common charges for atoms of the chemical elements. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Using a simple, general trend for the ionic charge for. Chlorine Net Charge.
From giodfhmom.blob.core.windows.net
Chlorine Liquid Cost at Matthew Delgado blog Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows updated on may 07, 2024. Net negative charge, the number of protons is less than the number of electrons. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons,. Chlorine Net Charge.
From www.vecteezy.com
Chlorine symbol. Chemical element of the periodic table. Vector Chlorine Net Charge 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. When writing the formula of an ionic compound we use the lowest. Using a simple, general trend for. Chlorine Net Charge.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Chlorine Net Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. There are two chloride ions in the formula. This table shows the most common charges for atoms of the chemical elements. 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Chlorine Net Charge.
From gioamsorw.blob.core.windows.net
Chlorine Ion Formed Name at Thomas Horton blog Chlorine Net Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. When writing the formula of an. Chlorine Net Charge.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Net Charge 93 rows updated on may 07, 2024. There are two chloride ions in the formula. When writing the formula of an ionic compound we use the lowest. Net negative charge, the number of protons is less than the number of electrons. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Although chlorine. Chlorine Net Charge.
From slideplayer.com
Illustrating the bonding of Sodium and Chlorine ppt download Chlorine Net Charge There are two chloride ions in the formula. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. When writing the formula of an ionic compound we use the lowest. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not. Chlorine Net Charge.
From www.chegg.com
Solved Chlorine forms an ion with a charge of? a. 7− b. 2+ Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. When writing the formula of an ionic compound we use the lowest. Net negative charge, the number of protons is less than the number of. Chlorine Net Charge.
From valenceelectrons.com
How many valence electrons does chlorine(Cl) have? Chlorine Net Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula.. Chlorine Net Charge.
From www.researchgate.net
Sketch comparing chlorination of ac) nickel and df) iron electrode at Chlorine Net Charge Net negative charge, the number of protons is less than the number of electrons. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. This table shows the most common charges for atoms of the. Chlorine Net Charge.
From giodfhmom.blob.core.windows.net
Chlorine Liquid Cost at Matthew Delgado blog Chlorine Net Charge When writing the formula of an ionic compound we use the lowest. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. 93 rows updated on may 07, 2024. There are two chloride ions in. Chlorine Net Charge.
From www.numerade.com
SOLVED (a) Determine the formal charge on the chlorine atom in the Chlorine Net Charge There are two chloride ions in the formula. This table shows the most common charges for atoms of the chemical elements. When writing the formula of an ionic compound we use the lowest. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge. Chlorine Net Charge.
From alchetron.com
Chlorine pentafluoride Alchetron, The Free Social Encyclopedia Chlorine Net Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. This table shows the most common charges for atoms of. Chlorine Net Charge.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Net Charge Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. When writing the formula of an ionic compound we use the lowest. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. There are two chloride ions in the formula. 93 rows updated on may. Chlorine Net Charge.
From gioamsorw.blob.core.windows.net
Chlorine Ion Formed Name at Thomas Horton blog Chlorine Net Charge Net negative charge, the number of protons is less than the number of electrons. When writing the formula of an ionic compound we use the lowest. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows updated on may 07, 2024. Although chlorine as an element is a diatomic molecule,. Chlorine Net Charge.