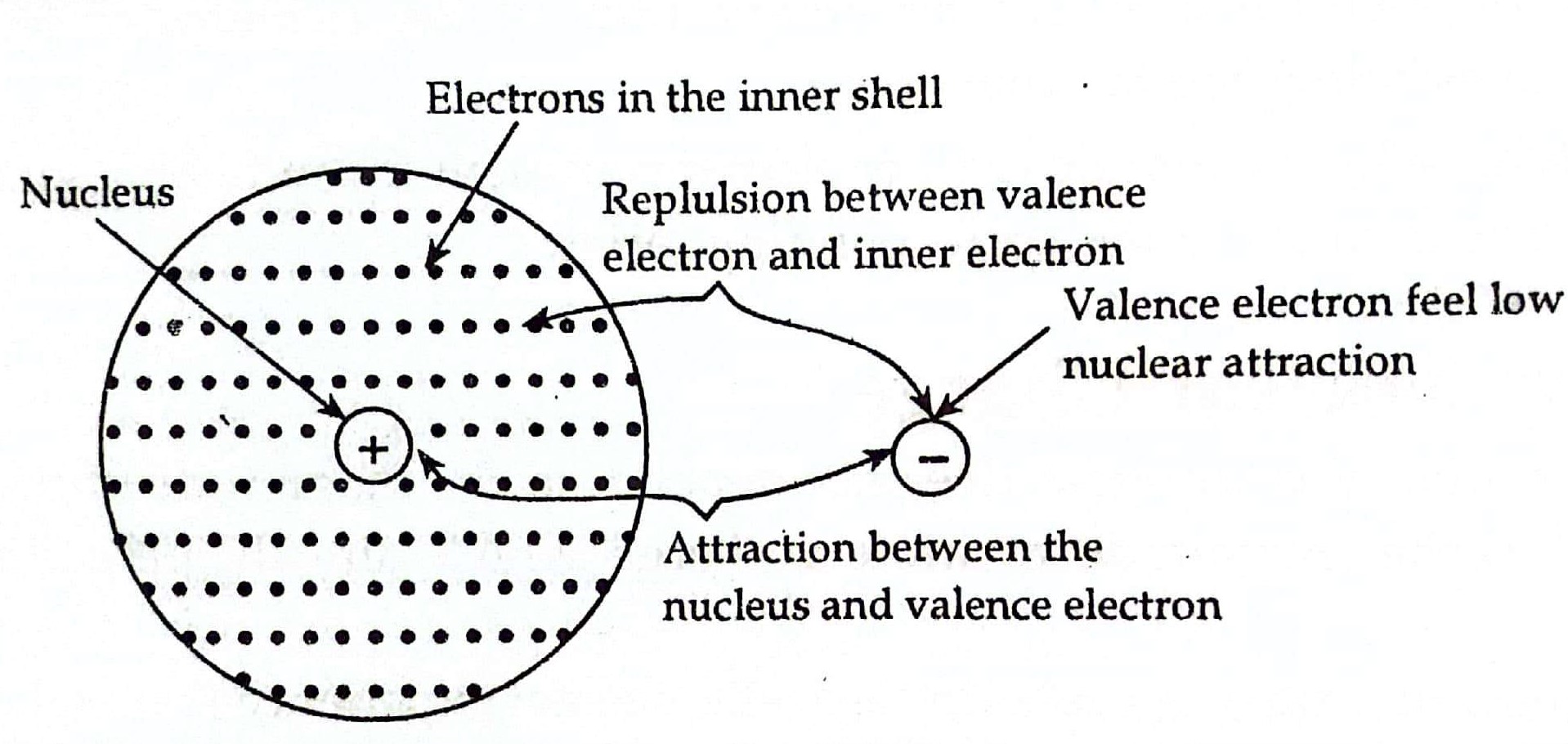
Which Statement About Electron Shielding Is False . Core electrons efficiently shield outermost electrons from nuclear. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. A it is easier to remove outer electrons to form cations because of the shielding effect. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Which statement about electron shielding is false? Which statement is true about electron shielding of nuclear charge? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. The balance between attractive and repulsive forces results in shielding. A it is easier to remove outer electrons to form cations because of the shielding effect. Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Which statement about electron shielding is false? The electrons are attracted to the nucleus at the same time as electrons repel each other.
from chemistnotes.com
The electrons are attracted to the nucleus at the same time as electrons repel each other. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Which statement about electron shielding is false? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Which statement is true about electron shielding of nuclear charge? The balance between attractive and repulsive forces results in shielding. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? A it is easier to remove outer electrons to form cations because of the shielding effect.
Shielding Effect or Screening Effect Definition, Factors Affecting
Which Statement About Electron Shielding Is False The balance between attractive and repulsive forces results in shielding. Which statement is true about electron shielding of nuclear charge? The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Which statement about electron shielding is false? Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. Which statement about electron shielding is false? Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Core electrons efficiently shield outermost electrons from nuclear. A it is easier to remove outer electrons to form cations because of the shielding effect. A it is easier to remove outer electrons to form cations because of the shielding effect. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. The electrons are attracted to the nucleus at the same time as electrons repel each other. The balance between attractive and repulsive forces results in shielding.
From kunduz.com
[ANSWERED] Which statement below regarding electron... Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Which statement about electron shielding is false? The balance between attractive and repulsive forces results in shielding. A it is easier to remove outer electrons to form cations because of the. Which Statement About Electron Shielding Is False.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Which Statement About Electron Shielding Is False The electrons are attracted to the nucleus at the same time as electrons repel each other. Which statement about electron shielding is false? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Which statement is true about electron shielding of nuclear charge? A it is easier to. Which Statement About Electron Shielding Is False.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Which Statement About Electron Shielding Is False Which statement is true about electron shielding of nuclear charge? A it is easier to remove outer electrons to form cations because of the shielding effect. Core electrons efficiently shield outermost electrons from nuclear. Which statement about electron shielding is false? Which statement about electron shielding is false? The electrons are attracted to the nucleus at the same time as. Which Statement About Electron Shielding Is False.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Which Statement About Electron Shielding Is False Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. A it is easier to remove outer electrons to form cations because of the shielding effect. A it is easier to. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Which of the following statements are true about Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Core electrons efficiently shield outermost electrons from nuclear. A it is easier to remove outer electrons to form cations because of the shielding effect. Which statement about electron shielding is false? The concept of electron shielding,. Which Statement About Electron Shielding Is False.
From chemistry.stackexchange.com
chemistry electron electron repulsion and shielding Which Statement About Electron Shielding Is False Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. Which statement about electron shielding is false? Study with quizlet and memorize flashcards containing terms like which statement. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED Classify each statement about effective nuclear charge, Zeff as Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Which statement is true about electron shielding of nuclear charge? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Core electrons efficiently shield. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED Classify each statement about and shielding as true Which Statement About Electron Shielding Is False The balance between attractive and repulsive forces results in shielding. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. A it is easier to remove outer electrons to. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Classify each statement about effective nuclear Which Statement About Electron Shielding Is False A it is easier to remove outer electrons to form cations because of the shielding effect. Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Core electrons efficiently shield outermost electrons from nuclear. Electron shielding is the process of partially shielding an electron from the attractive force. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved 9. Which statement is FALSE regarding the Lewis Which Statement About Electron Shielding Is False Core electrons efficiently shield outermost electrons from nuclear. A it is easier to remove outer electrons to form cations because of the shielding effect. Which statement about electron shielding is false? The balance between attractive and repulsive forces results in shielding. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. A it. Which Statement About Electron Shielding Is False.
From socratic.org
How are shielding effect and atomic radius related? Socratic Which Statement About Electron Shielding Is False Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. The electrons. Which Statement About Electron Shielding Is False.
From ar.inspiredpencil.com
Electron Shielding Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Core electrons efficiently shield outermost electrons from nuclear. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. A it is easier to remove outer electrons to form cations because of the shielding effect. A it is easier to remove outer electrons to. Which Statement About Electron Shielding Is False.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Which Statement About Electron Shielding Is False A it is easier to remove outer electrons to form cations because of the shielding effect. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. The electrons are attracted to the nucleus at the same time as electrons repel each other. Study with quizlet and memorize flashcards containing terms. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Classify each statement about effective nuclear Which Statement About Electron Shielding Is False Which statement about electron shielding is false? A it is easier to remove outer electrons to form cations because of the shielding effect. Which statement is true about electron shielding of nuclear charge? Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. The balance between attractive and repulsive forces. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Part E Which statement is true of the atom shown in Which Statement About Electron Shielding Is False A it is easier to remove outer electrons to form cations because of the shielding effect. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The balance between attractive and repulsive forces results in shielding. Electrons are negatively charged and are pulled pretty close to each other by their attraction. Which Statement About Electron Shielding Is False.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table Which Statement About Electron Shielding Is False Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Which statement about electron shielding is false? A it is easier to remove outer electrons to form cations because. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED Classify each statement about effective nuclear charge, Zeff as Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Core electrons efficiently shield outermost electrons from nuclear. Electron shielding. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED Classify each statement about effective nuclear charge, Zeff Which Statement About Electron Shielding Is False Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. Which statement is true about electron shielding of nuclear charge? A it is easier to remove outer electrons to form cations because of the shielding effect. Electron shielding is the process of partially shielding an electron from the attractive force. Which Statement About Electron Shielding Is False.
From kunduz.com
[ANSWERED] Choose CORRECT statement A Electron proton which are Kunduz Which Statement About Electron Shielding Is False Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The balance between attractive and repulsive forces results in shielding. Core electrons efficiently shield outermost electrons from nuclear. A it is easier to remove outer electrons to form cations because of the shielding effect. The concept of electron shielding, in which. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED "Which statement is true regarding Slater's rules and the Which Statement About Electron Shielding Is False Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. A it is easier to remove outer electrons to form cations because of the shielding effect. Study with quizlet. Which Statement About Electron Shielding Is False.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Which Statement About Electron Shielding Is False Core electrons efficiently shield outermost electrons from nuclear. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. The electrons are attracted to the nucleus at the same time as electrons repel each other. Which statement about electron shielding is false? The balance between attractive and. Which Statement About Electron Shielding Is False.
From www.studocu.com
Which statement is true about electron shielding of nuclear charge Which Statement About Electron Shielding Is False Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. Which statement about electron shielding is false? The balance between attractive and repulsive forces results in shielding. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Q10 Homework. Unanswered In the electron transport Which Statement About Electron Shielding Is False The electrons are attracted to the nucleus at the same time as electrons repel each other. Core electrons efficiently shield outermost electrons from nuclear. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved 30. Which statements completed with a e will be true Which Statement About Electron Shielding Is False Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The balance between attractive and repulsive forces results in shielding. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. A it is easier to remove outer. Which Statement About Electron Shielding Is False.
From www.youtube.com
Which one is a wrong statement?(a) Total orbital angular momentum of Which Statement About Electron Shielding Is False A it is easier to remove outer electrons to form cations because of the shielding effect. Which statement about electron shielding is false? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Core electrons efficiently shield outermost electrons from nuclear. The balance between attractive and repulsive forces results in shielding. Electrons are. Which Statement About Electron Shielding Is False.
From www.youtube.com
What is the basic difference between the terms electron gain enthalpy Which Statement About Electron Shielding Is False Core electrons efficiently shield outermost electrons from nuclear. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED Question 4 1 pts Which statement correctly explains why the Which Statement About Electron Shielding Is False Which statement about electron shielding is false? The electrons are attracted to the nucleus at the same time as electrons repel each other. Which statement is true about electron shielding of nuclear charge? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. A it is easier to. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Classify each statement about effective nuclear Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Which statement is true about electron shielding of nuclear charge? Which statement about electron shielding is false? Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. A it is easier to remove outer electrons to form cations because of the. Which Statement About Electron Shielding Is False.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Which Statement About Electron Shielding Is False A it is easier to remove outer electrons to form cations because of the shielding effect. Which statement about electron shielding is false? Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. A it is easier to remove outer electrons to form cations because of the shielding effect. Electron shielding refers to. Which Statement About Electron Shielding Is False.
From www.gkseries.com
Which of the following statement is false? Which Statement About Electron Shielding Is False Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? Core electrons efficiently shield outermost electrons from nuclear. Electron shielding is the process of partially shielding an electron from the attractive force of the nucleus. Electrons are negatively charged and are pulled pretty close to each other by their attraction to. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Which of the following statements is false? Electrons Which Statement About Electron Shielding Is False Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? The electrons are attracted to the nucleus at the same time as electrons repel each other. Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. Which statement is true about. Which Statement About Electron Shielding Is False.
From www.numerade.com
SOLVED The electron configuration for a sulfur atom is [Ne]3s^23p^4 Which Statement About Electron Shielding Is False Which statement about electron shielding is false? Electron shielding refers to the phenomenon where the attraction between an electron and the nucleus is reduced due to the. Electrons are negatively charged and are pulled pretty close to each other by their attraction to the positive charge of a nucleus. A it is easier to remove outer electrons to form cations. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved 4. Which of the following statements is true? A. Which Statement About Electron Shielding Is False Which statement is true about electron shielding of nuclear charge? Core electrons efficiently shield outermost electrons from nuclear. Which statement about electron shielding is false? A it is easier to remove outer electrons to form cations because of the shielding effect. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. Which Statement About Electron Shielding Is False.
From slideplayer.com
Effective Nuclear Charge, Shielding, and Trends on the Periodic Table Which Statement About Electron Shielding Is False Which statement about electron shielding is false? The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. Core electrons efficiently shield outermost electrons from nuclear. Which statement is true about electron shielding of nuclear charge? A it is easier to remove outer electrons to form cations. Which Statement About Electron Shielding Is False.
From www.chegg.com
Solved Which of the following is the best description of Which Statement About Electron Shielding Is False Which statement about electron shielding is false? The balance between attractive and repulsive forces results in shielding. Study with quizlet and memorize flashcards containing terms like which statement is true about electron shielding of nuclear charge? A it is easier to remove outer electrons to form cations because of the shielding effect. Electrons are negatively charged and are pulled pretty. Which Statement About Electron Shielding Is False.