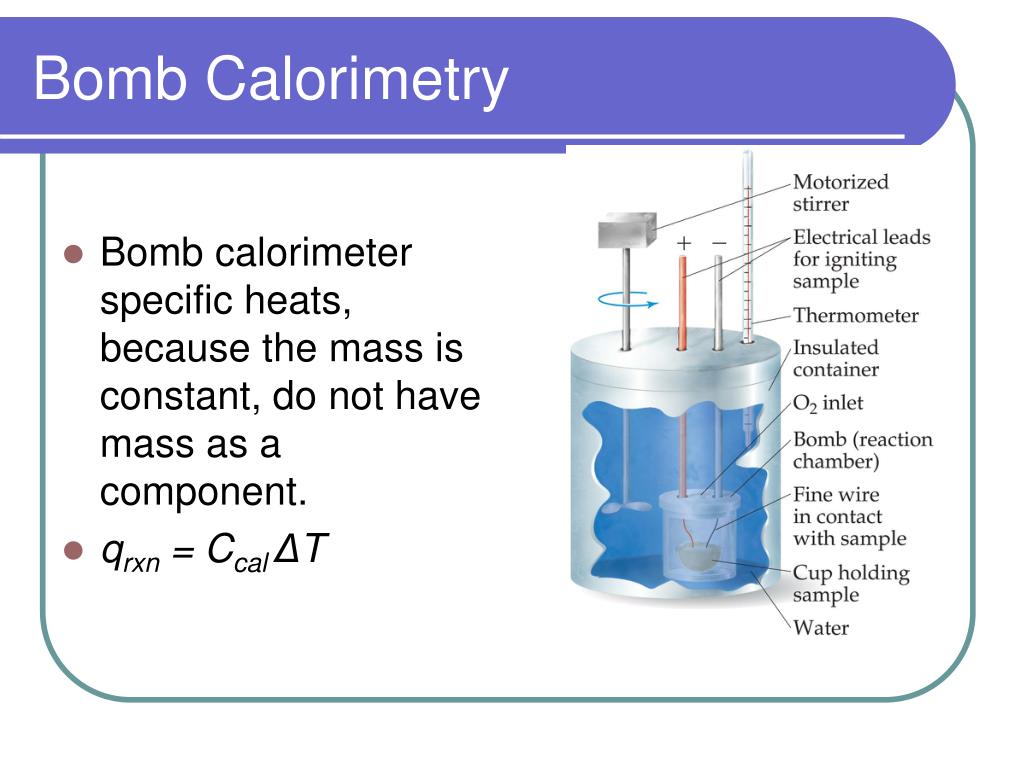
Bomb Calorimeter Disadvantages . It might seem like magic, but there’s a clever tool. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. No calorimeter is perfect because it can lose heat to its surroundings. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Constant volume calorimetry, also know. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and.
from www.slideserve.com
Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. Constant volume calorimetry, also know. No calorimeter is perfect because it can lose heat to its surroundings. Ever wondered exactly how scientists measure the heat content of things? Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure.
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation
Bomb Calorimeter Disadvantages No calorimeter is perfect because it can lose heat to its surroundings. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. No calorimeter is perfect because it can lose heat to its surroundings. Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Constant volume calorimetry, also know. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Disadvantages Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure.. Bomb Calorimeter Disadvantages.
From www.scribd.com
Bomb Calorimeter Principle,formula procedure.docx Calorimetry Enthalpy Bomb Calorimeter Disadvantages The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss.. Bomb Calorimeter Disadvantages.
From www.studypool.com
SOLUTION Bomb calorimeter ppt 1 Studypool Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Constant volume calorimetry, also know. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere. Bomb Calorimeter Disadvantages.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to. Bomb Calorimeter Disadvantages.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? No calorimeter is perfect because it can lose heat to its surroundings. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded. Bomb Calorimeter Disadvantages.
From studylib.net
STANDARD OPERATING PROCEDURE AND SAFETY GUIDE FOR BOMB CALORIMETER Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Constant volume calorimetry, also know. The adiabatic system, isothermal system, and the dry. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Disadvantages No calorimeter is perfect because it can lose heat to its surroundings. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. It might seem like magic, but there’s a clever tool. Constant volume calorimetry, also know. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are. Bomb Calorimeter Disadvantages.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. No calorimeter is perfect because it can lose heat to its surroundings. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. It might seem like magic, but there’s a. Bomb Calorimeter Disadvantages.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Ever wondered exactly how scientists measure the heat content. Bomb Calorimeter Disadvantages.
From 2012books.lardbucket.org
Calorimetry Bomb Calorimeter Disadvantages A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. No calorimeter is perfect because it can lose heat to its. Bomb Calorimeter Disadvantages.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Disadvantages The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. Constant volume calorimetry, also know. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all. Bomb Calorimeter Disadvantages.
From www.researchgate.net
Bomb Calorimeter Energy Balance Terms and Definitions Download Table Bomb Calorimeter Disadvantages Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure.. Bomb Calorimeter Disadvantages.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. No calorimeter is perfect because it can lose heat to its surroundings. Ever wondered exactly how scientists measure the heat content of things?. Bomb Calorimeter Disadvantages.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Disadvantages The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Constant volume calorimetry, also know. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Ever wondered exactly how scientists measure the heat content of things? Describe a simple calorimeter and explain how. Bomb Calorimeter Disadvantages.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. No calorimeter is perfect because it can lose heat to its surroundings. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Ever wondered exactly how scientists measure the heat content. Bomb Calorimeter Disadvantages.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Disadvantages Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. The calorimeter is made of austenitic steel which provides considerable. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT Bomb Calorimeter PowerPoint Presentation, free download ID3787850 Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Ever wondered exactly how scientists measure the heat content of things? No calorimeter is perfect because it can lose heat to its surroundings. The adiabatic system,. Bomb Calorimeter Disadvantages.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. Ever wondered exactly how scientists measure the heat content of things? No calorimeter is perfect because it can lose heat to its surroundings. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are. Bomb Calorimeter Disadvantages.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Disadvantages Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. No calorimeter is perfect because it can lose heat to its surroundings. Constant volume calorimetry, also know. The adiabatic system, isothermal system, and the dry. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT Bomb Calorimeters PowerPoint Presentation, free download ID4499873 Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Ever wondered exactly how scientists measure the heat content of things? It might seem like magic, but there’s a clever tool. The adiabatic. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT Q = MCΔT Specific Heat & CALORIMETRY PowerPoint Presentation Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample. Bomb Calorimeter Disadvantages.
From www.studypool.com
SOLUTION Bomb calorimeter revised Studypool Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. The calorimeter is made. Bomb Calorimeter Disadvantages.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Constant volume calorimetry, also know. A bomb calorimeter. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. It might seem like magic, but there’s a clever tool. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible. Bomb Calorimeter Disadvantages.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter Disadvantages The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Constant volume calorimetry, also know. It might seem like magic, but there’s a clever tool. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. A bomb calorimeter is used to measure, under. Bomb Calorimeter Disadvantages.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter Disadvantages Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Constant volume calorimetry, also know. It might seem like magic, but there’s a clever tool. Although bomb calorimeters in laboratories have insulation to minimize these loses,. Bomb Calorimeter Disadvantages.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. No calorimeter is perfect because it can lose heat to its surroundings. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel. Bomb Calorimeter Disadvantages.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. The adiabatic system, isothermal system, and the dry method (dry static jacket isothermal method) are compared and. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is. Bomb Calorimeter Disadvantages.
From www.studypool.com
SOLUTION Bomb calorimeter with examples instrument calculation and Bomb Calorimeter Disadvantages No calorimeter is perfect because it can lose heat to its surroundings. It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. The calorimeter is made of austenitic steel which provides. Bomb Calorimeter Disadvantages.
From www.slideserve.com
PPT Chemical Reactions / Thermochemistry / Gases PowerPoint Bomb Calorimeter Disadvantages No calorimeter is perfect because it can lose heat to its surroundings. Constant volume calorimetry, also know. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Describe a simple calorimeter and explain how it is employed and how its heat. Bomb Calorimeter Disadvantages.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. Although bomb calorimeters in laboratories have insulation to minimize these loses, it's impossible to stop all heat loss. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure.. Bomb Calorimeter Disadvantages.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Constant volume calorimetry, also know. Describe a simple calorimeter and explain how it is employed and how its heat capacity is. Bomb Calorimeter Disadvantages.
From www.chegg.com
Solved a) Calibration i. A bomb calorimeter filled with Bomb Calorimeter Disadvantages It might seem like magic, but there’s a clever tool. No calorimeter is perfect because it can lose heat to its surroundings. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Describe a simple calorimeter and explain how it is. Bomb Calorimeter Disadvantages.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Disadvantages No calorimeter is perfect because it can lose heat to its surroundings. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by. Bomb Calorimeter Disadvantages.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Disadvantages Constant volume calorimetry, also know. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Although bomb calorimeters in laboratories have. Bomb Calorimeter Disadvantages.