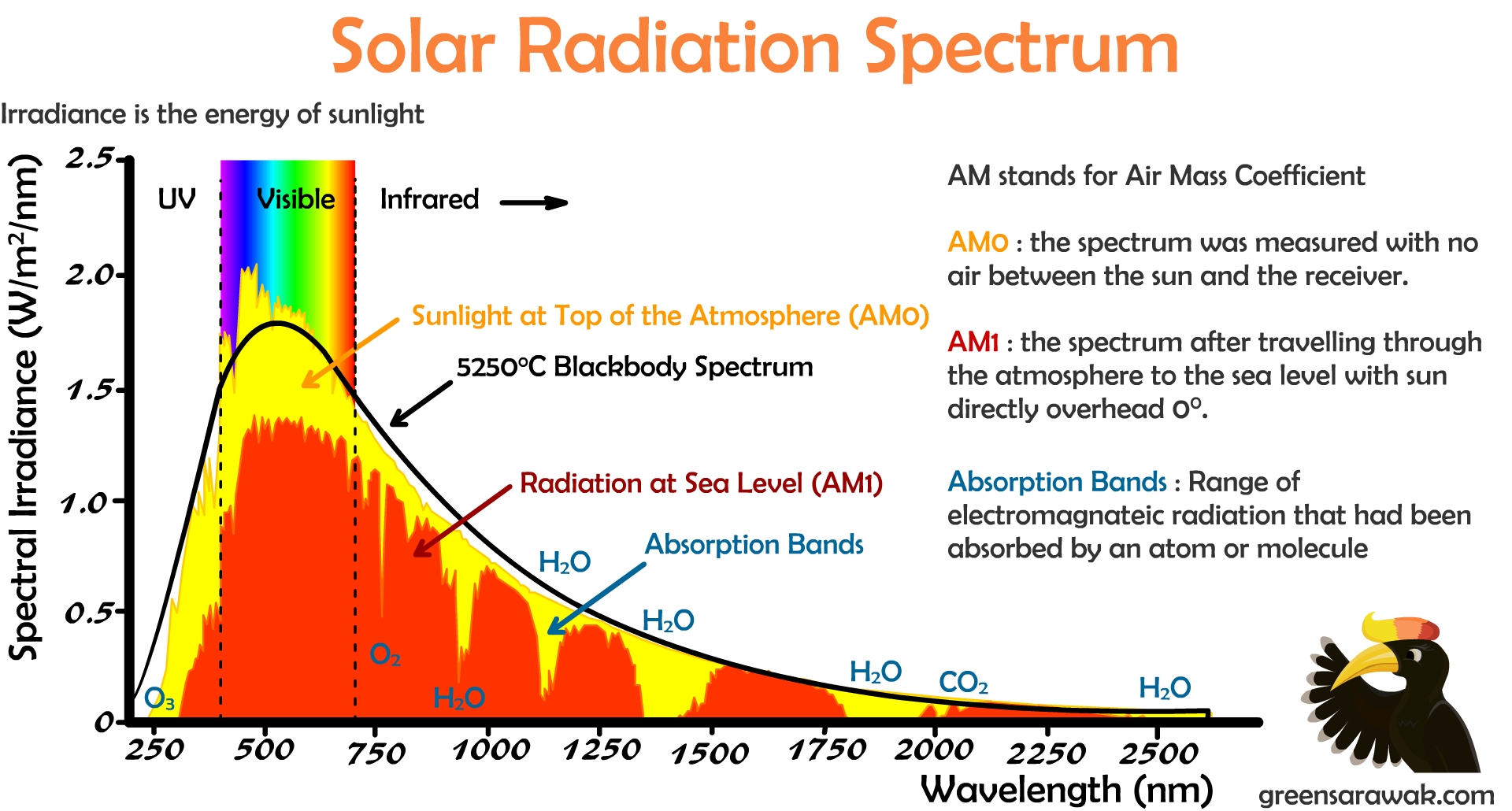
What Is Spectral Emission . We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. Why do we see emission lines when electrons return to the ground state? Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. What is an excited state? What is the ground state of an atom? The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. From spectral lines astronomers can determine not only the. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. Let’s go back to simple absorption and emission spectra. Atomic emission spectra are produced when excited electrons return to the ground state. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. What this object is depends on the scientific discipline. The science of spectroscopy is quite sophisticated. The emitted light of electrons corresponds to energies of the specific electrons.
from www.reddit.com
What is an excited state? The emitted light of electrons corresponds to energies of the specific electrons. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Why do we see emission lines when electrons return to the ground state? What this object is depends on the scientific discipline. Let’s go back to simple absorption and emission spectra. From spectral lines astronomers can determine not only the. What is the ground state of an atom? We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
Why is the sky not violet? r/askscience
What Is Spectral Emission Atomic emission spectra are produced when excited electrons return to the ground state. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. Atomic emission spectra are produced when excited electrons return to the ground state. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. From spectral lines astronomers can determine not only the. Let’s go back to simple absorption and emission spectra. The science of spectroscopy is quite sophisticated. What this object is depends on the scientific discipline. Why do we see emission lines when electrons return to the ground state? What is an excited state? What is the ground state of an atom? The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emitted light of electrons corresponds to energies of the specific electrons.
From users.highland.edu
Atomic Spectra and Models of the Atom What Is Spectral Emission Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectra are produced when excited electrons return to the ground state. What is the. What Is Spectral Emission.
From www.reddit.com
Why is the sky not violet? r/askscience What Is Spectral Emission What is an excited state? The science of spectroscopy is quite sophisticated. From spectral lines astronomers can determine not only the. What this object is depends on the scientific discipline. What is the ground state of an atom? Why do we see emission lines when electrons return to the ground state? Spectral emission refers to the process of emitting photons. What Is Spectral Emission.
From spiff.rit.edu
Spectrographs and Spectra What Is Spectral Emission We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. From spectral lines astronomers can determine not only the. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. When hydrogen gas is placed into a tube and electric current. What Is Spectral Emission.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations What Is Spectral Emission From spectral lines astronomers can determine not only the. What is an excited state? The science of spectroscopy is quite sophisticated. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. Atomic emission spectra are produced when excited electrons return to the ground state. Let’s go back to simple absorption and emission spectra.. What Is Spectral Emission.
From brokeasshome.com
periodic table emission spectra What Is Spectral Emission Atomic emission spectra are produced when excited electrons return to the ground state. What is an excited state? When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The science of spectroscopy is quite sophisticated. Why do we see emission lines when electrons return to the ground state? What. What Is Spectral Emission.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum What Is Spectral Emission Atomic emission spectra are produced when excited electrons return to the ground state. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. Let’s go back to simple absorption and emission spectra. The science of spectroscopy is quite sophisticated. Why do we see emission lines when electrons. What Is Spectral Emission.
From www.youtube.com
03.03 The Emission Spectrum of the Hydrogen Atom YouTube What Is Spectral Emission What is an excited state? Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. From spectral lines astronomers can determine not only the. When hydrogen. What Is Spectral Emission.
From www.researchgate.net
Emission spectral intensity and relative luminance of RPFs Download What Is Spectral Emission The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. From spectral lines astronomers can determine not only the. Atomic emission spectra are produced when excited electrons return to the ground state. When hydrogen gas is placed into a tube and electric current passed. What Is Spectral Emission.
From www.ptglab.com
Spectral Compensation in Flow Cytometry Proteintech Group What Is Spectral Emission Atomic emission spectra are produced when excited electrons return to the ground state. What this object is depends on the scientific discipline. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. The science of spectroscopy is quite sophisticated. The emitted light of electrons corresponds to energies of the specific electrons. Let’s go. What Is Spectral Emission.
From www.researchgate.net
Emission spectra of LEDs with different colors. Download Scientific What Is Spectral Emission What this object is depends on the scientific discipline. The emitted light of electrons corresponds to energies of the specific electrons. Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by. What Is Spectral Emission.
From pressbooks.online.ucf.edu
30.3 Bohr’s Theory of the Hydrogen Atom College Physics What Is Spectral Emission The science of spectroscopy is quite sophisticated. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. What this object is depends on the scientific discipline. Why do we see emission lines when electrons return to the ground state? Let’s go back to simple absorption and emission. What Is Spectral Emission.
From www.pinterest.com
The Red Shift of a Hydrogen Absorption Spectrum Wednesday, March 28 What Is Spectral Emission What is the ground state of an atom? From spectral lines astronomers can determine not only the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object.. What Is Spectral Emission.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts What Is Spectral Emission When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Let’s go back to simple absorption and emission spectra. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. From spectral lines astronomers can determine not only the. In chemistry, an emission. What Is Spectral Emission.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? What Is Spectral Emission What is the ground state of an atom? Why do we see emission lines when electrons return to the ground state? From spectral lines astronomers can determine not only the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The science of spectroscopy. What Is Spectral Emission.
From www.researchgate.net
The origins of three of the major sodium emission spectral lines (the What Is Spectral Emission The science of spectroscopy is quite sophisticated. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. What is an excited. What Is Spectral Emission.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum What Is Spectral Emission Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. The science of spectroscopy is quite sophisticated. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. We can use a star’s absorption spectrum to figure out what elements. What Is Spectral Emission.
From pixels.com
Helium Emission And Absorption Spectra Photograph by Carlos Clarivan What Is Spectral Emission Let’s go back to simple absorption and emission spectra. What is the ground state of an atom? The science of spectroscopy is quite sophisticated. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by. What Is Spectral Emission.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ What Is Spectral Emission What this object is depends on the scientific discipline. What is an excited state? Let’s go back to simple absorption and emission spectra. What is the ground state of an atom? The science of spectroscopy is quite sophisticated. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or. What Is Spectral Emission.
From www.esa.int
ESA Absorption and emission spectra of various elements What Is Spectral Emission In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Let’s go back to simple absorption and emission spectra. Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. We can use a star’s absorption spectrum. What Is Spectral Emission.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts What Is Spectral Emission When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emitted light of electrons corresponds to energies of the specific electrons. In. What Is Spectral Emission.
From www.thoughtco.com
Balmer Series Definition in Science What Is Spectral Emission The science of spectroscopy is quite sophisticated. From spectral lines astronomers can determine not only the. What this object is depends on the scientific discipline. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum. What Is Spectral Emission.
From hubblesite.org
Spectroscopy Reading the Rainbow What Is Spectral Emission In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. We can use a star’s absorption spectrum to figure out. What Is Spectral Emission.
From atmos.washington.edu
Radiation spectrum for the Sun and Earth. What Is Spectral Emission What is the ground state of an atom? Let’s go back to simple absorption and emission spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. We can use a star’s absorption spectrum to figure out what elements it is made of based. What Is Spectral Emission.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog What Is Spectral Emission What this object is depends on the scientific discipline. The science of spectroscopy is quite sophisticated. The emitted light of electrons corresponds to energies of the specific electrons. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. We can use a star’s absorption spectrum to figure out what elements it is made. What Is Spectral Emission.
From studylib.net
Every element has its own characteristic spectral emission What Is Spectral Emission Atomic emission spectra are produced when excited electrons return to the ground state. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. The science of spectroscopy is quite sophisticated.. What Is Spectral Emission.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction What Is Spectral Emission The science of spectroscopy is quite sophisticated. What is an excited state? What is the ground state of an atom? Atomic emission spectra are produced when excited electrons return to the ground state. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Spectral. What Is Spectral Emission.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps What Is Spectral Emission The emitted light of electrons corresponds to energies of the specific electrons. From spectral lines astronomers can determine not only the. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. We can use a star’s absorption spectrum to figure out what elements it is made. What Is Spectral Emission.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room What Is Spectral Emission The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. From spectral lines astronomers can determine not only the. Why do we see emission lines when electrons return to the ground state? Atomic emission spectra are produced when excited electrons return to the ground. What Is Spectral Emission.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum What Is Spectral Emission What this object is depends on the scientific discipline. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. Let’s go back to simple absorption and emission spectra. The science of spectroscopy is quite sophisticated. In chemistry, an emission spectrum refers to the range of wavelengths emitted. What Is Spectral Emission.
From geoengineering.global
Solar Radiation ManagementReflecting Sunlight to Cool the Climate What Is Spectral Emission Why do we see emission lines when electrons return to the ground state? Let’s go back to simple absorption and emission spectra. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. What this object is depends on the scientific discipline. In general, an emission spectrum. What Is Spectral Emission.
From infinitylearn.com
Line spectrum contains information about What Is Spectral Emission The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Why do we see emission lines when electrons return to the ground state? From spectral lines astronomers can determine not only the. The emitted light of electrons corresponds to energies of the specific electrons.. What Is Spectral Emission.
From www.researchgate.net
Relative spectral emission of (a) highpressure sodium (HPS) lamp What Is Spectral Emission From spectral lines astronomers can determine not only the. In general, an emission spectrum describes the wavelengths of the electromagnetic spectrum emitted by an energetic object. What is an excited state? Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. Atomic emission spectra are produced when excited electrons return. What Is Spectral Emission.
From www.sun.org
spectrum What Is Spectral Emission Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. What is an excited state? We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. The emitted light of electrons corresponds to energies of the specific electrons.. What Is Spectral Emission.
From laderarctic.weebly.com
Emission spectra laderarctic What Is Spectral Emission In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectra are produced when excited electrons return to the ground state. What is an excited state? The science of spectroscopy is quite sophisticated. What this object is depends on the scientific discipline. Why do. What Is Spectral Emission.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption What Is Spectral Emission We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it. What is the ground state of an atom? Spectral emission refers to the process of emitting photons of a specific frequency by a material, characterized by the spectral. Let’s go back to simple absorption and emission spectra.. What Is Spectral Emission.