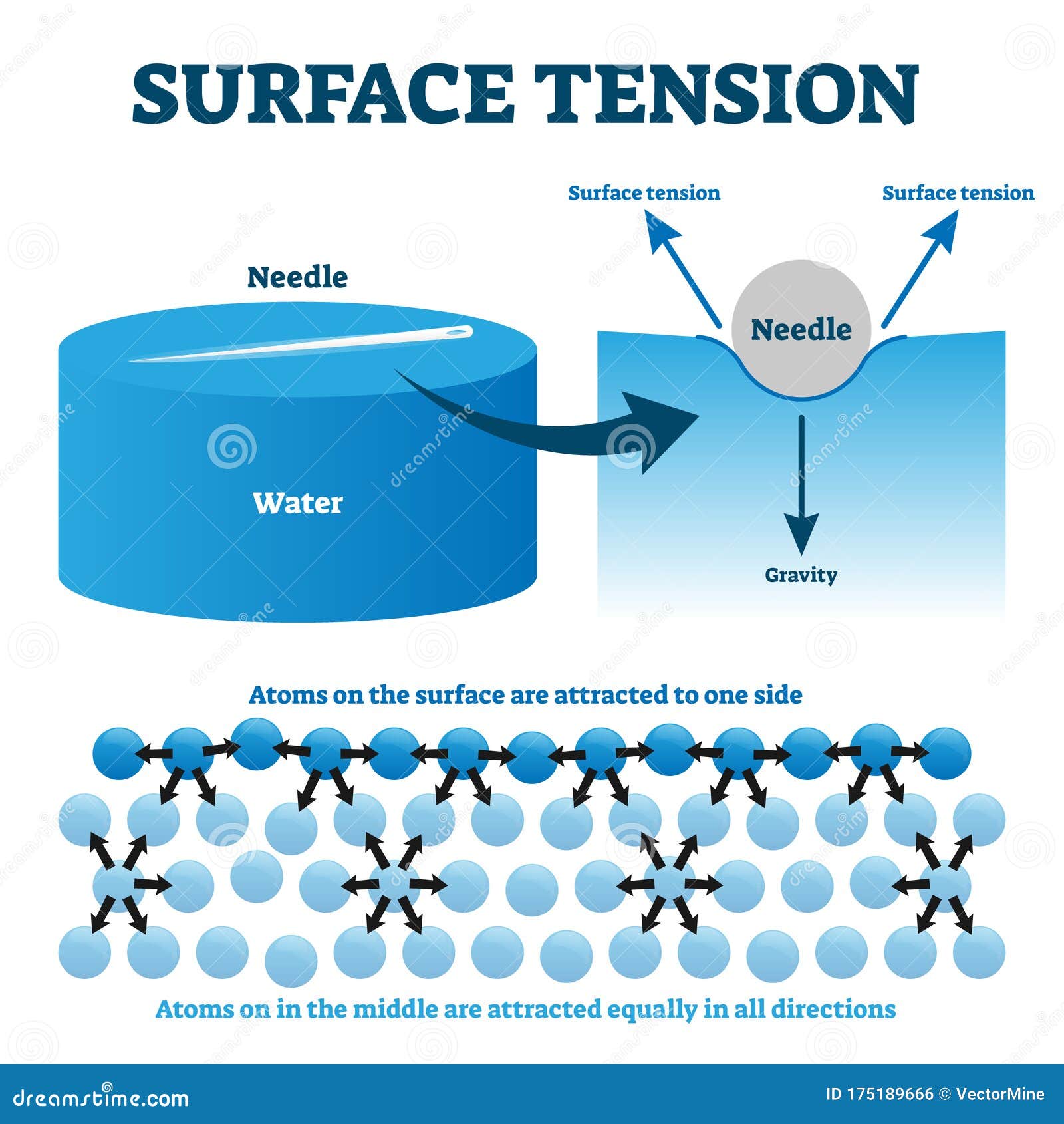
Surface Tension Of Liquids Quizlet . Surface tension is the reason why liquids form bubbles and droplets. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. It is often measured in joules per square meter (\ (j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. The inward surface tension force causes bubbles to be approximately spherical and raises the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,.
from www.dreamstime.com
Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the reason why liquids form bubbles and droplets.
Surface Tension Explanation Vector Illustration Diagram Stock Vector
Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the reason why liquids form bubbles and droplets. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. It is often measured in joules per square meter (\ (j/m^2\)). The inward surface tension force causes bubbles to be approximately spherical and raises the.
From www.toppr.com
The surface tension of a liquid is 5 N/m If a film is held on a ring of Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the reason why liquids form bubbles and droplets. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what. Surface Tension Of Liquids Quizlet.
From www.scribd.com
Explore The Concept of Surface Tension and Its Effects On Different Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the amount of energy required to increase. Surface Tension Of Liquids Quizlet.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry Surface Tension Of Liquids Quizlet It is often measured in joules per square meter (\ (j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The inward surface tension force causes bubbles to be approximately. Surface Tension Of Liquids Quizlet.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC Surface Tension Of Liquids Quizlet Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the. It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the amount of energy. Surface Tension Of Liquids Quizlet.
From www.studypool.com
SOLUTION Determination of Surface Tension of Liquids for B.sc(Hons Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the reason why liquids form bubbles and droplets. It is often measured in joules per square meter (\ (j/m^2\)). Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the amount. Surface Tension Of Liquids Quizlet.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. The inward surface tension force causes bubbles to be approximately spherical and raises the. It is often measured in joules per square meter (\ (j/m^2\)). Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what. Surface Tension Of Liquids Quizlet.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the reason why liquids form bubbles and droplets. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a liquid by a. Surface Tension Of Liquids Quizlet.
From engineeringcheatsheet.com
Surface Tension of Liquids Engineering Cheat Sheet Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension. Surface Tension Of Liquids Quizlet.
From fyoqevkxu.blob.core.windows.net
Surface Tension Quizlet at Lawrence Gardner blog Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Surface tension is the reason why. Surface Tension Of Liquids Quizlet.
From slideplayer.com
SURFACE TENSION OF A LIQUID for 1st sem ppt download Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. The inward surface tension force causes bubbles. Surface Tension Of Liquids Quizlet.
From almerja.com
Surface Tension Surface Tension Of Liquids Quizlet It is often measured in joules per square meter (\ (j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Surface tension is the amount of energy required to increase. Surface Tension Of Liquids Quizlet.
From www.youtube.com
Determination of surface tension of Liquid by using stalagmometer Surface Tension Of Liquids Quizlet Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The inward surface tension force causes bubbles to be approximately spherical and raises the. It is often measured in joules per. Surface Tension Of Liquids Quizlet.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the amount of energy required to increase the surface area. Surface Tension Of Liquids Quizlet.
From readchemistry.com
Determination of Surface Tension Read Chemistry Surface Tension Of Liquids Quizlet It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The inward surface tension force causes bubbles. Surface Tension Of Liquids Quizlet.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 Surface Tension Of Liquids Quizlet Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. Surface Tension Of Liquids Quizlet.
From www.chegg.com
Solved The surface tension of a liquid is being measured Surface Tension Of Liquids Quizlet Surface tension is the reason why liquids form bubbles and droplets. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface Tension Of Liquids Quizlet.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Surface tension is the reason why liquids form bubbles and droplets. Liquids that have strong intermolecular forces, like the. Surface Tension Of Liquids Quizlet.
From www.researchgate.net
Schemes of the main techniques for measuring surface tension of liquids Surface Tension Of Liquids Quizlet Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Surface tension, property of a liquid surface displayed by its acting as if it were. Surface Tension Of Liquids Quizlet.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Surface tension is the reason why liquids form bubbles and droplets. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid. Surface Tension Of Liquids Quizlet.
From printableangliavone.z21.web.core.windows.net
Give An Example Of Surface Tension Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. The surface tension of a liquid is a measure. Surface Tension Of Liquids Quizlet.
From www.dataphysics-instruments.com
Interfacial and surface tension of liquids explained DataPhysics Surface Tension Of Liquids Quizlet Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. It is often measured in joules per square meter (\ (j/m^2\)). Liquids that have strong intermolecular forces, like. Surface Tension Of Liquids Quizlet.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the reason why liquids form bubbles and droplets. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid. Surface Tension Of Liquids Quizlet.
From www.youtube.com
SRTMUN_BScFY_MECHANICSUNIT2_SURFACE TENSION_PART8Surface Tension of Surface Tension Of Liquids Quizlet Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. The. Surface Tension Of Liquids Quizlet.
From matmake.com
Surface Tension of Liquids Table Surface Tension Of Liquids Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the reason why liquids form bubbles and droplets. Study with quizlet and memorize flashcards containing terms like what does free. Surface Tension Of Liquids Quizlet.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Surface tension is the. Surface Tension Of Liquids Quizlet.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension Of Liquids Quizlet Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. It is often measured in joules per square meter (\ (j/m^2\)). Study with quizlet and. Surface Tension Of Liquids Quizlet.
From www.meritnation.com
Surface tension acting on the liquid depends upon mass of the liquid Surface Tension Of Liquids Quizlet Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The inward. Surface Tension Of Liquids Quizlet.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy. Surface Tension Of Liquids Quizlet.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the reason why liquids form bubbles and droplets. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. It is often measured in joules per square meter (\. Surface Tension Of Liquids Quizlet.
From www.doubtnut.com
The surface tension of a liquid is affected by Surface Tension Of Liquids Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. It is. Surface Tension Of Liquids Quizlet.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation Surface Tension Of Liquids Quizlet It is often measured in joules per square meter (\ (j/m^2\)). The inward surface tension force causes bubbles to be approximately spherical and raises the. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the amount of energy required to increase the surface area of a liquid by a. Surface Tension Of Liquids Quizlet.
From www.semanticscholar.org
[PDF] How Is the Surface Tension of Various Liquids Distributed along Surface Tension Of Liquids Quizlet The inward surface tension force causes bubbles to be approximately spherical and raises the. It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Surface tension is the reason why liquids form bubbles and droplets. Surface tension, property of. Surface Tension Of Liquids Quizlet.
From www.studocu.com
Determining the Surface Tension of Liquid by Capillary Rise Method Surface Tension Of Liquids Quizlet Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the. Surface Tension Of Liquids Quizlet.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Tension Of Liquids Quizlet It is often measured in joules per square meter (\ (j/m^2\)). Surface tension is the reason why liquids form bubbles and droplets. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Liquids. Surface Tension Of Liquids Quizlet.
From www.pinterest.com
Exploration of Liquids Surface Tension Resource Preview Surface Surface Tension Of Liquids Quizlet Study with quizlet and memorize flashcards containing terms like what does free surface of liquid consist of?, what is surface tension force,. The inward surface tension force causes bubbles to be approximately spherical and raises the. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like. Surface Tension Of Liquids Quizlet.