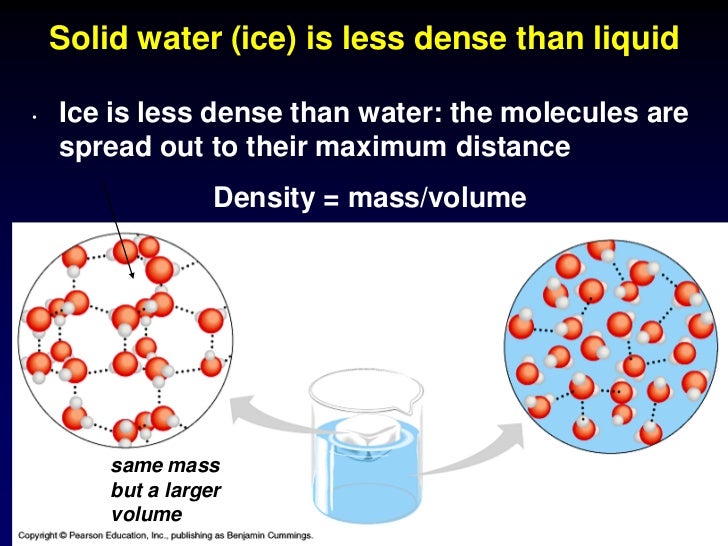
Water Density Vs Ice Density . If ice sank to the. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. A layer of ice forms, but. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. This means ice floats on water. The particles in a solid are tightly packed, so a lot of particles fit into a given space. The density of ice is less than water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ponds or lakes begin to freeze at the surface, closer to the cold air. Water has a higher density than air. Ice is less dense than liquid water and so it floats. Ice is less dense than liquid water. Ice floats because it is about 9% less dense than liquid water.
from www.slideshare.net
Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Ice floats because it is about 9% less dense than liquid water. This means ice floats on water. Ponds or lakes begin to freeze at the surface, closer to the cold air. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. If ice sank to the. The density of ice is less than water. Ice is less dense than liquid water. Water has a higher density than air.
Lecture 9. properties of water
Water Density Vs Ice Density Ice floats because it is about 9% less dense than liquid water. If ice sank to the. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. This means ice floats on water. The density of ice is less than water. Ice floats because it is about 9% less dense than liquid water. The particles in a solid are tightly packed, so a lot of particles fit into a given space. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Water has a higher density than air. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. A layer of ice forms, but. Ponds or lakes begin to freeze at the surface, closer to the cold air. Ice is less dense than liquid water and so it floats. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid.
From nicholasbsullivan.com
Images and Figures for Oceanography Water Density Vs Ice Density This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Water has a higher density than air. The density of ice is less than water. Ponds or lakes begin to. Water Density Vs Ice Density.
From student-tutor.com
Understanding the Density of Water StudentTutor Education Blog Water Density Vs Ice Density Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Ice is less dense than liquid water. Water has a higher density than air. Ice is. Water Density Vs Ice Density.
From www.researchgate.net
Density variation of ice and water across the phase transition. The Water Density Vs Ice Density This is due to the water expanding as it is frozen because of the hydrogen forming an open type. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are. Water Density Vs Ice Density.
From canvolf.weebly.com
Density of ice vs water canvolf Water Density Vs Ice Density The particles in a solid are tightly packed, so a lot of particles fit into a given space. Ice is less dense than liquid water and so it floats. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. If ice sank to the. Water has a higher. Water Density Vs Ice Density.
From mungfali.com
Density Of Water Chart Water Density Vs Ice Density The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. This means ice floats on water. Ice is less dense than liquid water. If ice sank to the. Ice floats because it is about 9% less dense than liquid water. A layer of ice forms, but. The. Water Density Vs Ice Density.
From www.slideserve.com
PPT Properties of Ice I PowerPoint Presentation, free download ID Water Density Vs Ice Density A layer of ice forms, but. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. Water is unusual in. Water Density Vs Ice Density.
From www.thoughtco.com
Why Is Water More Dense Than Ice? Water Density Vs Ice Density The density of ice is less than water. Ice is less dense than liquid water. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. Ice is less dense than liquid water and so it floats. Water has a higher density than air.. Water Density Vs Ice Density.
From www.slideserve.com
PPT Chemistry of Water PowerPoint Presentation, free download ID Water Density Vs Ice Density The particles in a solid are tightly packed, so a lot of particles fit into a given space. Ponds or lakes begin to freeze at the surface, closer to the cold air. A layer of ice forms, but. This means ice floats on water. Ice is less dense than liquid water and so it floats. Water is unusual in that. Water Density Vs Ice Density.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Water Density Vs Ice Density Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. This means ice floats on water. Ice is less dense than liquid water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Ponds. Water Density Vs Ice Density.
From www.slideshare.net
Lecture 9. properties of water Water Density Vs Ice Density If ice sank to the. Ice floats because it is about 9% less dense than liquid water. A layer of ice forms, but. Ice is less dense than liquid water. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. In other words, ice takes up about. Water Density Vs Ice Density.
From www.metoffice.gov.uk
Sea ice an overview Met Office Water Density Vs Ice Density Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Water has a higher density than air. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. Ponds or lakes begin to freeze at the surface, closer to the cold air. This. Water Density Vs Ice Density.
From lakeice.squarespace.com
Lake Ice Getting Ready Water Density Vs Ice Density Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. If ice sank to the. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. This means ice floats on water. Ponds or lakes begin to. Water Density Vs Ice Density.
From www.expii.com
Unique Densities (Water) — Properties & Examples Expii Water Density Vs Ice Density This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. A layer of ice forms, but. This means ice floats on water. Since water has maximum density at 4°c, water at that temperature sinks. Water Density Vs Ice Density.
From www.youtube.com
DENSITY OF ICE YouTube Water Density Vs Ice Density This means ice floats on water. The density of ice is less than water. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ponds or lakes begin to freeze at the surface, closer to the cold air. A layer of ice forms, but. Ice floats because it is. Water Density Vs Ice Density.
From openlab.citytech.cuny.edu
Water physical properties Biology 1101 Course Hub Water Density Vs Ice Density Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. The particles in a solid are tightly packed, so a lot of particles fit. Water Density Vs Ice Density.
From www.youtube.com
comparison of water vs Ice density theoryAtharva Online study centre Water Density Vs Ice Density Ice is less dense than liquid water. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. Ice is less dense than liquid water and so it floats. This means ice floats on water. The density of ice is less than water. Ponds. Water Density Vs Ice Density.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Water Density Vs Ice Density The density of ice is less than water. Ice is less dense than liquid water and so it floats. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Water has a higher density than air. In other words, ice takes up about 9% more space than water, so. Water Density Vs Ice Density.
From studylib.net
Density of Ice vs. Water Water Density Vs Ice Density If ice sank to the. Ponds or lakes begin to freeze at the surface, closer to the cold air. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. The particles in a solid. Water Density Vs Ice Density.
From www.toppr.com
Density of ice is ρ and that of water is σ . What will be the decrease Water Density Vs Ice Density If ice sank to the. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ice is less dense than liquid water. The particles in a solid are tightly packed, so a lot of particles fit into a given space. Ice floats because it is about 9% less dense. Water Density Vs Ice Density.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC Water Density Vs Ice Density Ice is less dense than liquid water and so it floats. A layer of ice forms, but. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ice floats because it is about 9% less dense than liquid water. This is due to the water expanding as it is. Water Density Vs Ice Density.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download Water Density Vs Ice Density The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Ponds or lakes begin to freeze at the surface, closer to the cold air. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Water. Water Density Vs Ice Density.
From www.slideserve.com
PPT LIFE DEPENDS ON THE UNIQUE PROPERITIES OF WATER PowerPoint Water Density Vs Ice Density The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Ice is less dense than liquid water. Ice floats because it is about 9% less dense than liquid water. Water has a higher density than air. Ice is less denser than water because in ice the molecules. Water Density Vs Ice Density.
From husbynv.weebly.com
Densitet Naturvetenskap och teknik Water Density Vs Ice Density This means ice floats on water. The density of ice is less than water. Water has a higher density than air. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it. Water is unusual in that its maximum density occurs as a liquid, rather than as a. Water Density Vs Ice Density.
From www.worldatlas.com
Do Water And Ice Weigh The Same? WorldAtlas Water Density Vs Ice Density Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved. Water Density Vs Ice Density.
From assemeister.weebly.com
assemeister Blog Water Density Vs Ice Density Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. The density of water is roughly 1 gram per milliliter but, this changes with temperature or. Water Density Vs Ice Density.
From www.slideserve.com
PPT Phase Diagram for CO 2 PowerPoint Presentation, free download Water Density Vs Ice Density The density of ice is less than water. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. If ice sank to the. Water has a higher density than air. Ponds or lakes begin to freeze at the surface, closer to the cold air. The particles in a solid are tightly packed, so. Water Density Vs Ice Density.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii Water Density Vs Ice Density Water has a higher density than air. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. The density of ice is less than water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Ice is less denser than water because in ice. Water Density Vs Ice Density.
From edu.rsc.org
The density of ice Resource RSC Education Water Density Vs Ice Density In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ice is less dense than liquid water and so it floats. The density of ice is less. Water Density Vs Ice Density.
From www.wikihow.com
How to Find the Density of Water 10 Steps (with Pictures) Water Density Vs Ice Density Ice is less dense than liquid water and so it floats. This means ice floats on water. The particles in a solid are tightly packed, so a lot of particles fit into a given space. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all. Water Density Vs Ice Density.
From www.geeksforgeeks.org
Density of Water Factors, Temperature Scales, Examples, FAQs Water Density Vs Ice Density The particles in a solid are tightly packed, so a lot of particles fit into a given space. If ice sank to the. This means ice floats on water. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Since water has maximum density at 4°c, water at that. Water Density Vs Ice Density.
From www.researchgate.net
1. Density of ice as function of temperature Download Scientific Diagram Water Density Vs Ice Density Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Water has a higher density than air. Ice is less dense than liquid water and so it floats. Ice is less dense than liquid water. This is due to the water expanding as it is frozen because of the. Water Density Vs Ice Density.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation ID2739092 Water Density Vs Ice Density The particles in a solid are tightly packed, so a lot of particles fit into a given space. Water has a higher density than air. A layer of ice forms, but. Ice is less dense than liquid water and so it floats. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure. Water Density Vs Ice Density.
From www.geeksforgeeks.org
What is the Density of Water? Formula, Factors, Examples & FAQs Water Density Vs Ice Density In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice is less dense than liquid water and so it floats. The density of ice is less than water. A layer of ice forms, but. This is due to the water expanding as it is frozen because of. Water Density Vs Ice Density.
From www.slideserve.com
PPT Properties of Ice I PowerPoint Presentation, free download ID Water Density Vs Ice Density Ice is less dense than liquid water and so it floats. The particles in a solid are tightly packed, so a lot of particles fit into a given space. Ponds or lakes begin to freeze at the surface, closer to the cold air. Since water has maximum density at 4°c, water at that temperature sinks to the bottom of a. Water Density Vs Ice Density.
From tukioka-clinic.com
🎉 What is the density of water at 20 degrees celsius. Density of Water Water Density Vs Ice Density If ice sank to the. Water is unusual in that its maximum density occurs as a liquid, rather than as a solid. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. This is due to the water expanding as it is frozen because of the hydrogen forming. Water Density Vs Ice Density.