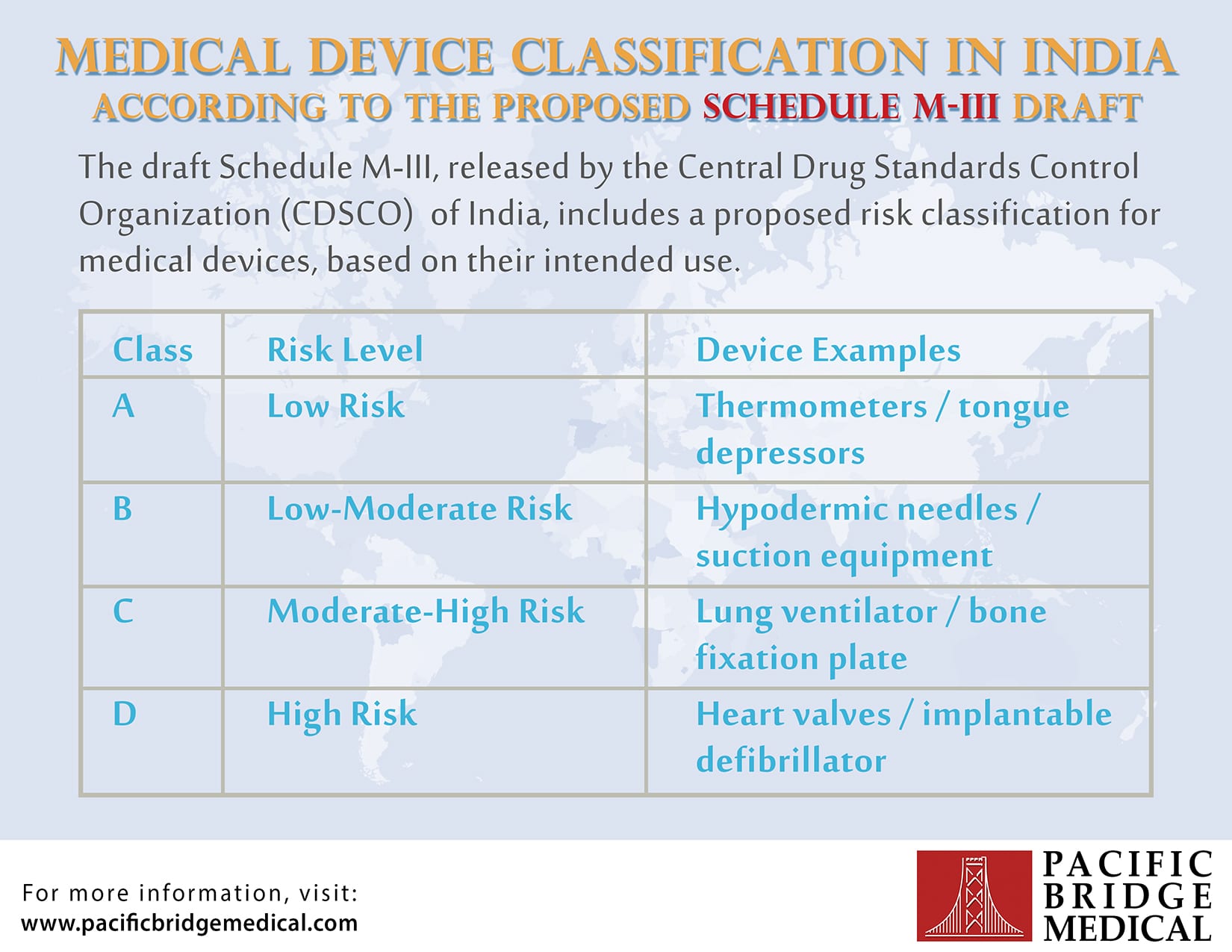
Medical Device Classification India . the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. In india, all medical devices are regulated under the drugs & cosmetics. 89 rows medical device. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. medical device rules, 2017 are applicable to: s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. 409(e)_amendment in rule 18 and 19 for.
from www.pacificbridgemedical.com
medical device rules, 2017 are applicable to: 409(e)_amendment in rule 18 and 19 for. In india, all medical devices are regulated under the drugs & cosmetics. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. 89 rows medical device. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,.
Device Classification in India Infographic
Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 409(e)_amendment in rule 18 and 19 for. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device rules, 2017 are applicable to: Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. In india, all medical devices are regulated under the drugs & cosmetics. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. 89 rows medical device. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,.
From mavink.com
Medical Device Classification Chart Medical Device Classification India medical device rules, 2017 are applicable to: in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical device. the indian classification system is based. Medical Device Classification India.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Classification India medical device rules, 2017 are applicable to: in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. In india, all medical devices are regulated under the drugs & cosmetics. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. 89 rows medical device. 409(e)_amendment in. Medical Device Classification India.
From www.electronicsforu.com
Challenges Faced In Designing Approved Medical Devices In India Medical Device Classification India in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. medical device rules, 2017 are applicable to: In india, all medical devices are regulated under the drugs & cosmetics. 409(e)_amendment in rule 18 and 19. Medical Device Classification India.
From www.tuvsud.com
Infographic The New Medical Device Regulation TÜV SÜD in India Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 409(e)_amendment in rule 18 and 19 for. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. 89 rows medical device. s.no title release date download pdf pdf size 1. Medical Device Classification India.
From www.thermofisher.com
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Medical Device Classification India medical device rules, 2017 are applicable to: (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. In india, all medical devices are regulated under the drugs. Medical Device Classification India.
From www.corpseed.com
Classification of Medical Devices by CDSCO in India Corpseed Medical Device Classification India the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. 409(e)_amendment in rule 18 and 19 for. medical device rules, 2017 are applicable to: ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical device. Substances used for. Medical Device Classification India.
From www.vrogue.co
Classification Of Medical Devices vrogue.co Medical Device Classification India s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. In india, all medical devices are regulated under the drugs & cosmetics. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. the indian. Medical Device Classification India.
From mungfali.com
Classification Of Medical Devices Medical Device Classification India in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. 89 rows medical device. ministry of health & family welfare, government of india has released the. Medical Device Classification India.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification India In india, all medical devices are regulated under the drugs & cosmetics. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. medical device rules, 2017 are applicable to: in india, clinical investigation is required for all class b, c, and d medical devices if the device is an. Medical Device Classification India.
From www.vrogue.co
A Primer On Medical Device Classification vrogue.co Medical Device Classification India In india, all medical devices are regulated under the drugs & cosmetics. 409(e)_amendment in rule 18 and 19 for. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. 89 rows medical device. the indian classification system is based on the mdr 2017 guidance and are. Medical Device Classification India.
From rs-ness.com
IVD Classification Under IVDR Definition RS NESS Medical Device Classification India (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the indian classification system is based on the mdr 2017. Medical Device Classification India.
From www.vrogue.co
Device Classification In India Infographic vrogue.co Medical Device Classification India the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device rules, 2017 are applicable to: s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. in india,. Medical Device Classification India.
From english.nmpa.gov.cn
Rules for Classification of Medical Devices Medical Device Classification India (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. 409(e)_amendment in rule 18 and 19 for. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 89 rows medical device. In india, all medical devices are regulated under the drugs & cosmetics. medical device rules,. Medical Device Classification India.
From www.researchgate.net
Medical Device Classification a Download Scientific Diagram Medical Device Classification India the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 89 rows medical device. 409(e)_amendment in rule 18 and 19 for. (r) “custom made medical device” means a medical device made specifically in accordance with a written. Medical Device Classification India.
From mavink.com
Mdr Classification Chart Medical Device Classification India medical device rules, 2017 are applicable to: 409(e)_amendment in rule 18 and 19 for. In india, all medical devices are regulated under the drugs & cosmetics. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 89 rows medical device. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. ministry of health &. Medical Device Classification India.
From www.qualtechs.com
INDIA Issuance of a draft copy of new “Guidance Document for Medical Medical Device Classification India In india, all medical devices are regulated under the drugs & cosmetics. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. medical device rules, 2017 are applicable to: (r) “custom made medical device” means. Medical Device Classification India.
From www.vrogue.co
Device Classification In India Infographic vrogue.co Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. 409(e)_amendment in rule 18 and 19 for. medical device rules, 2017 are applicable to: (r) “custom made medical device” means a. Medical Device Classification India.
From www.vrogue.co
Device Classification In India Infographic vrogue.co Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. medical device rules, 2017 are applicable to: In india, all medical devices are regulated under the drugs & cosmetics. 409(e)_amendment in rule 18 and 19 for. in india, clinical. Medical Device Classification India.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Device Classification India 89 rows medical device. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 409(e)_amendment in rule 18 and 19 for. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. the indian. Medical Device Classification India.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification India medical device rules, 2017 are applicable to: in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. 409(e)_amendment. Medical Device Classification India.
From operonstrategist.com
CDSCO Manufacturing License Get Manufacturing License for Medical Medical Device Classification India in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. medical device rules, 2017 are applicable to: 89 rows medical device. In india, all medical devices are regulated under the drugs & cosmetics. . Medical Device Classification India.
From www.vrogue.co
Device Classification In India Infographic vrogue.co Medical Device Classification India Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. In india, all medical devices are regulated under the drugs & cosmetics. in india, clinical investigation is required for all class b, c, and d medical devices if the. Medical Device Classification India.
From operonstrategist.com
India's New IVD Device Classification Enhances Patient Safety and Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical device. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. medical device rules, 2017 are. Medical Device Classification India.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Classification India (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. 409(e)_amendment in rule 18 and 19 for. in india, clinical. Medical Device Classification India.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Medical Device Classification India (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. 409(e)_amendment in rule 18 and 19 for. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an. Medical Device Classification India.
From www.youtube.com
Indian MDR 2017 Medical Device Classification YouTube Medical Device Classification India 89 rows medical device. medical device rules, 2017 are applicable to: 409(e)_amendment in rule 18 and 19 for. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. the indian classification system is. Medical Device Classification India.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification India in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. In india, all medical devices are regulated under the drugs & cosmetics. 89 rows medical device. ministry of health & family welfare, government of. Medical Device Classification India.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Classification India Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 409(e)_amendment in rule 18 and 19 for. In india, all medical devices are regulated under the drugs & cosmetics. in india, clinical investigation is required for all class b, c,. Medical Device Classification India.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 56 OFF Medical Device Classification India the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. 409(e)_amendment in rule 18 and 19 for. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device rules, 2017 are applicable to: s.no title release date download pdf pdf. Medical Device Classification India.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Classification India the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of. Medical Device Classification India.
From cliniexperts.com
Medical Device Grouping as per MDR 2017 CliniExperts CliniExperts Medical Device Classification India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 409(e)_amendment in rule 18 and 19 for. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. the indian classification system is based on the mdr 2017 guidance. Medical Device Classification India.
From www.tuvsud.com
India Medical Device Regulations TÜV SÜD Medical Device Classification India s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. In india, all medical devices are regulated under the drugs & cosmetics. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 409(e)_amendment in rule 18 and 19 for.. Medical Device Classification India.
From www.meddeviceonline.com
Medical Devices Regulatory Priorities In India Medical Device Classification India In india, all medical devices are regulated under the drugs & cosmetics. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. s.no title release date download pdf pdf size 1 2023.06.02_mdr_final g.s.r. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. (r) “custom. Medical Device Classification India.
From www.infoholicresearch.com
Classification Of Newly Notified Medical Devices In India Infoholic Medical Device Classification India medical device rules, 2017 are applicable to: (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. in india, clinical investigation is required for all class b, c, and d medical devices if the device is an. Medical Device Classification India.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification India in india, clinical investigation is required for all class b, c, and d medical devices if the device is an investigational. Substances used for in vitro diagnosis and surgical dressings, surgical bandages,. the indian classification system is based on the mdr 2017 guidance and are dependent on the intended use,. medical device rules, 2017 are applicable to:. Medical Device Classification India.