Calorimetry Prelab . Calorimetry is used to measure amounts of heat transferred to. Experiment 25 prelaboratory assignment calorimetry date desk no. To apply these \( \delta h\) values in a hess’s. Calculate the molar enthalpy of dissolution in kj/mol. Use hess’s law to determine the enthalpy of combustion of a. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. What is the procedure for. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter.
from www.studocu.com
Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Experiment 25 prelaboratory assignment calorimetry date desk no. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. To apply these \( \delta h\) values in a hess’s. Calculate the molar enthalpy of dissolution in kj/mol. Use hess’s law to determine the enthalpy of combustion of a. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. What is the procedure for.
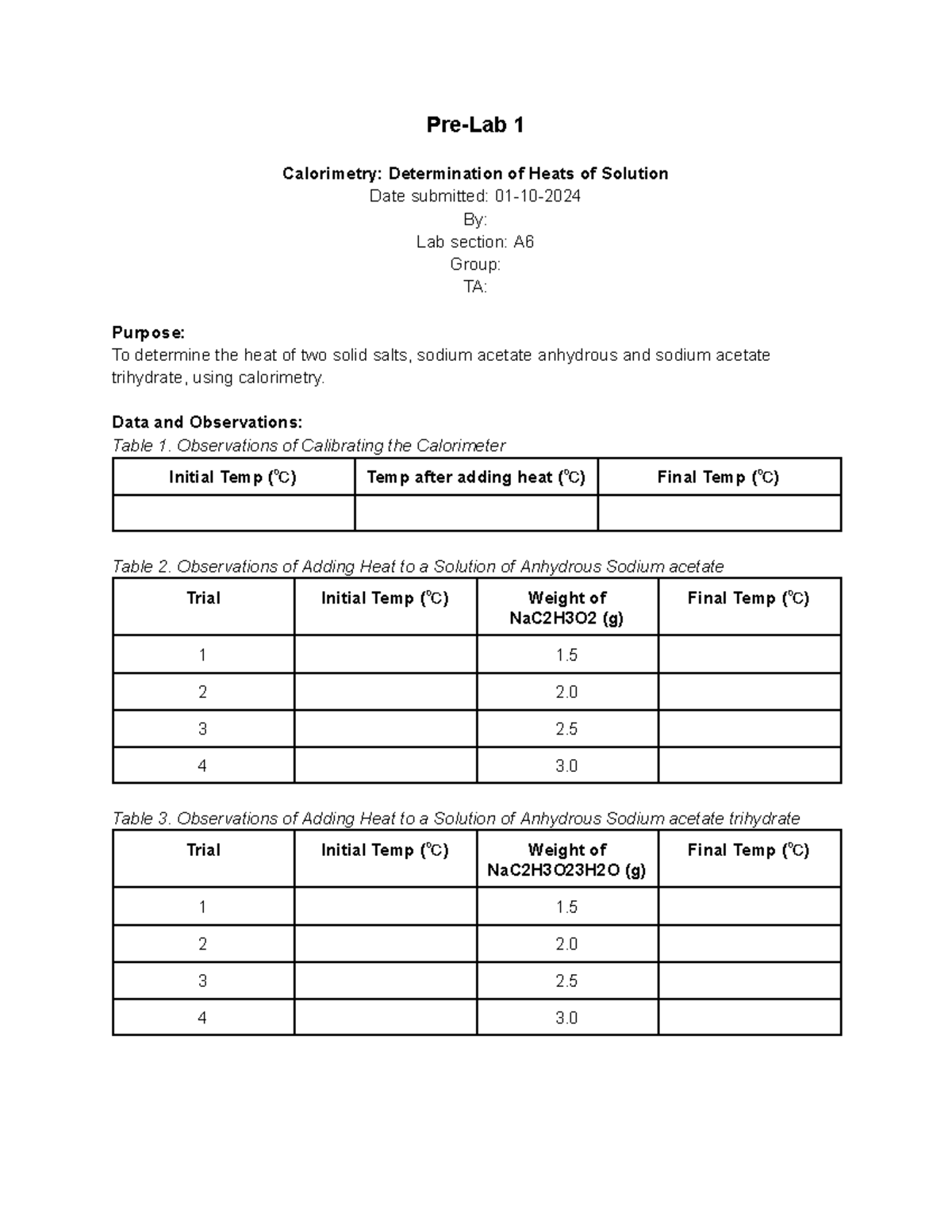
Calorimetry Prelab PreLab 1 Calorimetry Determination of Heats of
Calorimetry Prelab Calorimetry is used to measure amounts of heat transferred to. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Use hess’s law to determine the enthalpy of combustion of a. Calorimetry is used to measure amounts of heat transferred to. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. What is the procedure for. Calculate the molar enthalpy of dissolution in kj/mol. To apply these \( \delta h\) values in a hess’s. Experiment 25 prelaboratory assignment calorimetry date desk no. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry.
From www.youtube.com
Calorimetry prelab YouTube Calorimetry Prelab Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a. Calorimetry Prelab.
From www.studocu.com
Calorimetry prelab pre lab Calorimetry Determination of heats of Calorimetry Prelab Calorimetry is used to measure amounts of heat transferred to. Calculate the molar enthalpy of dissolution in kj/mol. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. To apply these \( \delta h\) values in a hess’s. What is the heat, q, in joules transferred by. Calorimetry Prelab.
From www.studocu.com
Cal pre lab Calorimetry pre lab Calorimetry Determination of Heats Calorimetry Prelab Calculate the molar enthalpy of dissolution in kj/mol. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. What is the procedure for. To apply these \( \delta h\) values in a hess’s. To experimentally measure the \( \delta h\) values of two reactions using. Calorimetry Prelab.
From www.chegg.com
Solved Prelab for Experiment 2A Solution Calorimetry In Calorimetry Prelab Experiment 25 prelaboratory assignment calorimetry date desk no. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. What is the procedure for. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Calorimetry is used to measure. Calorimetry Prelab.
From www.studocu.com
UTF8''Prelabbomb calorimetry Chemistry 122 Laboratory Bomb Calorimetry Prelab To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To apply these \( \delta h\) values in a hess’s.. Calorimetry Prelab.
From www.youtube.com
Calorimetry Prelab Lecture YouTube Calorimetry Prelab What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In calorimetry, an insulated reaction container, called a calorimeter,. Calorimetry Prelab.
From www.chegg.com
Solved CHM 2045L Heat Effects and Calorimetry Prelab Heat Calorimetry Prelab What is the procedure for. Calculate the molar enthalpy of dissolution in kj/mol. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Calorimetry is used to measure amounts of heat transferred to. Use hess’s law to determine the enthalpy of combustion of a. A. Calorimetry Prelab.
From www.chegg.com
Solved Heat Flow and Calorimetry Prelab Questions Section Calorimetry Prelab To apply these \( \delta h\) values in a hess’s. Calculate the molar enthalpy of dissolution in kj/mol. Use hess’s law to determine the enthalpy of combustion of a. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot. Calorimetry Prelab.
From www.youtube.com
Prelab 2 Calorimetry and Heat of Reaction YouTube Calorimetry Prelab A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Calculate the molar enthalpy of dissolution in kj/mol. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. What is the procedure for. In calorimetry, an insulated reaction container, called a calorimeter, is. Calorimetry Prelab.
From www.chegg.com
COMMNITY cOUrGE Heat Flow and Calorimetry Prelab Calorimetry Prelab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Use hess’s law to determine the enthalpy of combustion of a. Experiment 6 ∙ calorimetry 6‐3 consider what. Calorimetry Prelab.
From www.chegg.com
Solved Date Name Lab Section Prelab Assignment Calorimetry Prelab Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. To apply these \( \delta h\) values in a hess’s. What is the heat, q, in. Calorimetry Prelab.
From www.studocu.com
Calorimetry Prelab CALORIMETRY DETERMINATION OF HEATS OF SOLUTION Calorimetry Prelab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry Prelab.
From www.youtube.com
Prelab 3 Calorimetry YouTube Calorimetry Prelab Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. To apply these \( \delta h\) values in a hess’s. Use hess’s law to determine the enthalpy of combustion of a. To experimentally measure the \( \delta h\) values of two reactions using the technique. Calorimetry Prelab.
From www.chegg.com
Solved Name Prelab 2 Calorimetry Measuring the Energy Calorimetry Prelab Calorimetry is used to measure amounts of heat transferred to. Use hess’s law to determine the enthalpy of combustion of a. To apply these \( \delta h\) values in a hess’s. What is the procedure for. Experiment 25 prelaboratory assignment calorimetry date desk no. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant. Calorimetry Prelab.
From www.stuvia.com
Chem 103 Solution Calorimetry PreLab Chem 103 Stuvia US Calorimetry Prelab Use hess’s law to determine the enthalpy of combustion of a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate the molar enthalpy of dissolution in kj/mol. Calorimetry is used to measure amounts of heat transferred to. To experimentally measure the \( \delta h\) values of. Calorimetry Prelab.
From www.studocu.com
Calorimetry/Cal prelab template Calorimetry Determination of Heats Calorimetry Prelab What is the procedure for. To apply these \( \delta h\) values in a hess’s. Experiment 25 prelaboratory assignment calorimetry date desk no. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Calculate the molar enthalpy of dissolution in kj/mol. To experimentally measure the \( \delta. Calorimetry Prelab.
From www.studocu.com
Calorimetry Part 2 prelab Riley McConaughey Chem 202 1 821832161 Lab Calorimetry Prelab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Use hess’s law to determine the enthalpy of combustion of a. Calculate the molar enthalpy of dissolution in kj/mol. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry.. Calorimetry Prelab.
From www.chegg.com
Solved Name Prelab 2 Calorimetry Measuring the Energy Calorimetry Prelab To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. To apply these \( \delta h\) values in a. Calorimetry Prelab.
From www.chegg.com
Solved Prelab Calorimetry Name > A calorimeter cup is made Calorimetry Prelab Calculate the molar enthalpy of dissolution in kj/mol. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. Experiment 25 prelaboratory assignment calorimetry date desk no. What is. Calorimetry Prelab.
From www.studocu.com
Potato Chip Calorimetry Lab Prelab Questions Name Calorimetry Prelab Experiment 25 prelaboratory assignment calorimetry date desk no. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In calorimetry, an insulated reaction container, called a calorimeter, is filled with. Calorimetry Prelab.
From www.chegg.com
Solved Calorimetry Experiment Prelab 1. Calculate the Calorimetry Prelab What is the procedure for. Calorimetry is used to measure amounts of heat transferred to. Experiment 25 prelaboratory assignment calorimetry date desk no. Use hess’s law to determine the enthalpy of combustion of a. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. In calorimetry, an insulated reaction container, called. Calorimetry Prelab.
From www.chegg.com
Solved Calorimeter Prelab Define Specific Heat. 2) In what Calorimetry Prelab Use hess’s law to determine the enthalpy of combustion of a. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. Experiment 25 prelaboratory assignment calorimetry date desk no. In calorimetry, an insulated reaction container, called a. Calorimetry Prelab.
From www.studocu.com
Cal prelab Cal CALORIMETRY DETERMINATION OF HEATS OF SOLUTION Date Calorimetry Prelab Calculate the molar enthalpy of dissolution in kj/mol. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A 5.85 g sample of. Calorimetry Prelab.
From www.studocu.com
CalPreLab chem 1002 Calorimetry Determination of Heats of Solutions Calorimetry Prelab What is the procedure for. Calorimetry is used to measure amounts of heat transferred to. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. Calculate the molar enthalpy of dissolution in kj/mol. Experiment 25 prelaboratory assignment. Calorimetry Prelab.
From studylib.net
Heat Capacity of Metals PreLab Calorimetry Prelab What is the procedure for. Calculate the molar enthalpy of dissolution in kj/mol. To apply these \( \delta h\) values in a hess’s. Experiment 25 prelaboratory assignment calorimetry date desk no. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. What is the heat, q, in joules. Calorimetry Prelab.
From www.studocu.com
2 Calorimetry Prelab(Exp2) Chem 120, Summer Quarter 2011 Page 1 of 2 Calorimetry Prelab To apply these \( \delta h\) values in a hess’s. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Use hess’s law to determine the enthalpy of combustion of a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Prelab.
From www.studocu.com
Calorimetry 2 prelab Chem 200/ Calorimetry Part 2 Enthalpy of a Calorimetry Prelab Experiment 25 prelaboratory assignment calorimetry date desk no. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. What is the procedure for. Calorimetry is used to measure amounts of heat transferred to. Use hess’s law to determine the enthalpy of combustion of a. Calculate the molar enthalpy of dissolution in. Calorimetry Prelab.
From www.youtube.com
Calorimetry Prelab Talk, 32 minutes YouTube Calorimetry Prelab What is the procedure for. Use hess’s law to determine the enthalpy of combustion of a. Experiment 25 prelaboratory assignment calorimetry date desk no. Calculate the molar enthalpy of dissolution in kj/mol. Calorimetry is used to measure amounts of heat transferred to. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid. Calorimetry Prelab.
From www.chegg.com
Solved PRELAB ASSIGNMENT 1. What is a calorimeter? What is Calorimetry Prelab To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. To apply these \( \delta h\) values in a hess’s. Calculate the molar enthalpy of dissolution in kj/mol. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. What is. Calorimetry Prelab.
From www.chegg.com
Solved CHM 2045L Heat Effects and Calorimetry Prelab Heat Calorimetry Prelab Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. Calculate the. Calorimetry Prelab.
From www.coursehero.com
[Solved] Chemistry prelab thank you! Prelab Exp 2 Coffee Cup Calorimetry Prelab What is the procedure for. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Experiment 25 prelaboratory assignment calorimetry date desk no. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is. Calorimetry Prelab.
From www.chegg.com
Prelab Assignment Calorimetry and Hess's Law 1. Show Calorimetry Prelab Use hess’s law to determine the enthalpy of combustion of a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To apply these \( \delta h\) values in a hess’s. What is the procedure for. What is the heat, q, in joules transferred by a chemical reaction. Calorimetry Prelab.
From www.chegg.com
Solved Heat Flow and Calorimetry Prelab Questions sections Calorimetry Prelab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Calorimetry is used to measure amounts of heat transferred to. What is. Calorimetry Prelab.
From www.studocu.com
Calorimetry Prelab PreLab 1 Calorimetry Determination of Heats of Calorimetry Prelab Calorimetry is used to measure amounts of heat transferred to. To apply these \( \delta h\) values in a hess’s. Calculate the molar enthalpy of dissolution in kj/mol. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Use hess’s law to determine the enthalpy of combustion of. Calorimetry Prelab.
From www.chegg.com
Solved CHM 2045L Heat Effects and Calorimetry Prelab Heat Calorimetry Prelab Calculate the molar enthalpy of dissolution in kj/mol. What is the heat, q, in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 115 g of dilute aqueous solution (c=4.184 j/g ⋅ °c) if the reaction causes. A 5.85 g sample of a salt with a molar mass of 84.10 g/mol is added to a calorimeter.. Calorimetry Prelab.