What Is Electron Probability Distribution . As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's.
from www.theochem.ru.nl
If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability.
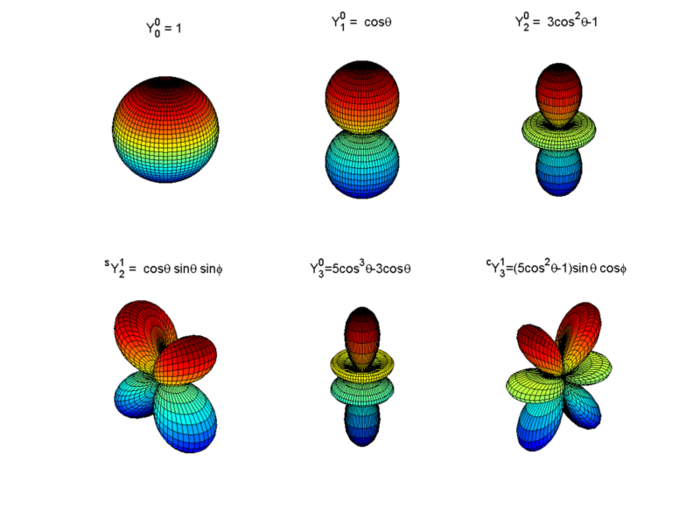
Spherical harmonics Knowino
What Is Electron Probability Distribution We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability.
From quantitative-probabilitydistribution.blogspot.com
Probability Distribution Curve For 1s Research Topics What Is Electron Probability Distribution For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the. What Is Electron Probability Distribution.
From www.researchgate.net
(a)(d) The electron probability density Q\left({\rho }_{1},{\rho What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. For each orbital, its radial density distribution describes the regions with particular. What Is Electron Probability Distribution.
From www.slideserve.com
PPT Atomic Orbitals PowerPoint Presentation, free download ID2011734 What Is Electron Probability Distribution For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. We can obtain an energy and one or more wave functions for every value. What Is Electron Probability Distribution.
From www.chegg.com
Solved Here is a sketch of the radial probability What Is Electron Probability Distribution We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. If we know their energies, which we do, then the best we. What Is Electron Probability Distribution.
From chem.libretexts.org
Chapter 2.5 Atomic Orbitals and Their Energies Chemistry LibreTexts What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around. What Is Electron Probability Distribution.
From www.slideserve.com
PPT Atomic Orbitals PowerPoint Presentation, free download ID2011734 What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. We can obtain an energy and one or more wavefunctions for every value of n n, the principal. What Is Electron Probability Distribution.
From brainly.com
The radial probability distribution for an electron in an atom a What Is Electron Probability Distribution For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. If we know their energies, which we do, then the best we can do is to calculate a. What Is Electron Probability Distribution.
From brainly.in
Draw radial probability distribution curves for 3s and 4d orbitals What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. We can obtain an energy and one or more wave functions for every value of n, the. What Is Electron Probability Distribution.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. We can obtain an energy and one or more wavefunctions for every value of. What Is Electron Probability Distribution.
From www.researchgate.net
Electronpair probability distribution functions for the ∞ He, 4 He, H What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. We can obtain an energy and one or more wavefunctions for every value of. What Is Electron Probability Distribution.
From www.researchgate.net
(a)(c) The electron probability density Q\left({\rho }_{1},{\rho What Is Electron Probability Distribution For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but. What Is Electron Probability Distribution.
From www.theochem.ru.nl
Spherical harmonics Knowino What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. As one way of graphically representing the probability distribution, the probability of finding an. What Is Electron Probability Distribution.
From www.meritnation.com
Draw the radial probability distribution curves for 2s and 2p electron What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is. What Is Electron Probability Distribution.
From www.slideserve.com
PPT Molecular Orbitals PowerPoint Presentation, free download ID What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around. What Is Electron Probability Distribution.
From nigerianscholars.com
Probability Distribution Introduction to Quantum Physics What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. If we know their energies, which we do, then the best we can do is to calculate a probability distribution. What Is Electron Probability Distribution.
From www.researchgate.net
The electron probability density in the xz plane,... Download What Is Electron Probability Distribution We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. To determine the probability of finding an electron in a hydrogen atom in a particular region of space,. What Is Electron Probability Distribution.
From www.slideserve.com
PPT Atomic Structure & Periodicity PowerPoint Presentation, free What Is Electron Probability Distribution If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. Repeated measurements on identical atoms will produce interesting probability distributions. What Is Electron Probability Distribution.
From brainly.com
(a) What is the probability of an electron state being filled if it is What Is Electron Probability Distribution Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number,. What Is Electron Probability Distribution.
From www.youtube.com
Quantum Mechanical Model (Part 3 of 9) Electron Probability Density What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. If we know their energies, which we do, then the best we. What Is Electron Probability Distribution.
From www.researchgate.net
Electron probability densities of H + 2 at the end of the attosecond What Is Electron Probability Distribution Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. As one way of graphically representing the probability distribution, the probability of finding an electron is. What Is Electron Probability Distribution.
From www.researchgate.net
Top Probability distribution for H − , a two electron system. Bottom What Is Electron Probability Distribution If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. We can obtain an energy and one or more wavefunctions. What Is Electron Probability Distribution.
From byjus.com
The probability density curve for 2s electron appears like What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the. What Is Electron Probability Distribution.
From www.researchgate.net
Electron probability distributions obtained by projecting the What Is Electron Probability Distribution We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by. What Is Electron Probability Distribution.
From www.reddit.com
This so the probability distribution diagram for Hydrogen atom's What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. If we know their energies, which we do, then the best we can do. What Is Electron Probability Distribution.
From www.researchgate.net
Probability distribution of the electrons in the neon atom (Source What Is Electron Probability Distribution As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. If we know their energies, which we do, then the best we can do. What Is Electron Probability Distribution.
From www.researchgate.net
Relative probability density distributions of the electron pair being What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. We can obtain an energy and one or more wave functions for every value of n, the principal quantum. What Is Electron Probability Distribution.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry What Is Electron Probability Distribution If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. Repeated measurements on identical atoms will produce interesting probability distributions for electrons. What Is Electron Probability Distribution.
From socratic.org
What is the meaning and explanation of each equation? ( wave function What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. To determine the probability of finding an electron in a hydrogen atom in a particular. What Is Electron Probability Distribution.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom Probability Of Finding An Electron In A Hydrogen Atom What Is Electron Probability Distribution Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. We can obtain an energy and one or more wave functions for every value of n, the principal quantum number, by solving schrödinger's. We can obtain an energy and one or more wavefunctions for every value of n n, the principal. What Is Electron Probability Distribution.
From www.researchgate.net
Radial distribution probability densities D(r) = r 2 R(r) 2 for 4f, 5s What Is Electron Probability Distribution To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. For each orbital, its radial density distribution describes the regions with particular probabilities for. What Is Electron Probability Distribution.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry What Is Electron Probability Distribution Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is. What Is Electron Probability Distribution.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is Electron Probability Distribution Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they will not. We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. We can obtain an energy and one or more wave functions for every value of n, the principal quantum. What Is Electron Probability Distribution.
From www.researchgate.net
Electron probability distributions obtained by projecting the What Is Electron Probability Distribution For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. As one way of graphically representing the probability distribution, the probability of finding an electron is indicated by the density of. We can obtain an energy and one or more wavefunctions for every value of n n, the principal. What Is Electron Probability Distribution.
From www.numerade.com
SOLVED Question 3 The FermiDirac distribution function (f(E)) gives What Is Electron Probability Distribution We can obtain an energy and one or more wavefunctions for every value of n n, the principal quantum number, by solving. For each orbital, its radial density distribution describes the regions with particular probabilities for finding an electron in that particular orbital. Repeated measurements on identical atoms will produce interesting probability distributions for electrons around the atom, but they. What Is Electron Probability Distribution.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts What Is Electron Probability Distribution If we know their energies, which we do, then the best we can do is to calculate a probability distribution that describes the likelihood of. To determine the probability of finding an electron in a hydrogen atom in a particular region of space, it is necessary to integrate the probability. We can obtain an energy and one or more wave. What Is Electron Probability Distribution.